Home Education & Research PKC

Trop-2-dependent Recruitment of PKCα to the Cell Membrane
Marco Trerotola and Saverio Alberti
The TROP2 gene (TACSTD2) (1,2) encodes a transmembrane calcium signal transducer (3), that is characterized by a non-canonical EGF-like repeat and by a thyroglobulin domain (1,4-6). The Trop-2 EGF-like and thyroglobulin domains demonstrate significant similarity to the corresponding regions of Trop-1/Ep-CAM, nidogen and IGF-binding proteins (1,4,5). The TROP2 gene is highly conserved across species (1,4), consistently with a strong evolutionary pressure for a conserved functional role.
PKCα is activated in a Trop-2-dependent manner. As activated PKCα translocates to the cell membrane (7), a Trop-2-dependent recruitment of PKCα to the cell membrane was visualized by confocal time-lapse microscopy analysis of living MTE 4-14 cells transfected with Trop-2-mRFP1 and with PKCα–EGFP. Trop-2-mRFP1 mainly localized at the cell membrane rim, in the endoplasmic reticulum and in vesicular formations, as in natively expressing cancer cells. Dynamic colocalization of PKCα with Trop-2 in membrane ruffles and podosomes was shown (Movie 1). Recursive waves of strikingly coordinated relocation of PKCα and Trop-2 to specific domains of the cell membrane were observed every few to several minutes, morphological analysis suggesting a tight relationship with podosome dynamics (Movie 1). No such changes were detected in control cells transfected with PKCδ–EGFP (Movie 2).
Material and methods
Cells
The murine immortalized MTE 4-14 cell line (8) was maintained in DMEM supplemented with 10% fetal calf serum.
Plasmids
The pEYFP expression vector was obtained from Clontech (Palo Alto, CA). The pGFP3-PKC-α-WT and pGFP3-PKC-δ-WT expression vectors were generously provided by Jae-Won Soh (9). The expression vector pmRFP1 (10) was kindly provided by R. Tsien. Chimeric proteins between Trop-2 and mRFP1 were constructed as described (11,12), after substituting the mRFP1 (XhoI / KpnI) coding region for that of EYFP in pEYFP.
Confocal time-lapse microscopy
Cells cultured on glass slides were incubated in Leibovitz’s F15 culture medium devoid of phenol red and bicarbonate, supplemented with 10% FBS, 50 UI/ml Penicillin/Streptomycin and 2 mM N-acetyl-cysteine (Sigma) to reduce free-radical damage. Cells were viewed with an LSM-510 META (Zeiss) confocal microscope. Images were captured at 1 min intervals. Excitation of EGFP was at 488 nm; mRFP1 was excited at 546 nm.
Movie legends
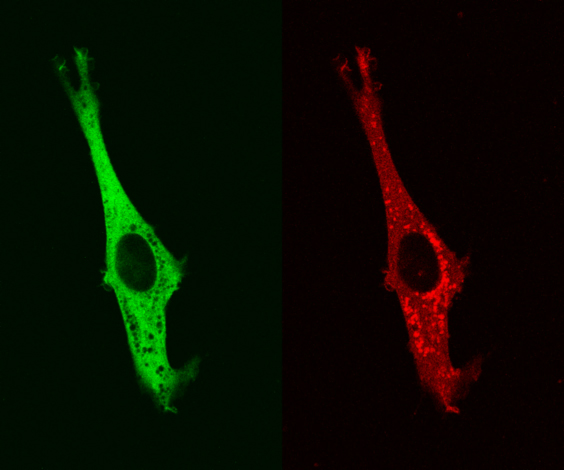
Movie 1 – MTE 4-14 cells transfected with Trop-2-mRFP1 and with PKCα–EGFP. Cytoplasmic vescicles containing Trop-2-mRFP1 are useful landmarks for the localization of PKCα–EGFP (empty cytoplasmic regions). Trop-2-mRFP1 and PKCα–EGFP dynamically colocalize in membrane ruffles and podosomes.
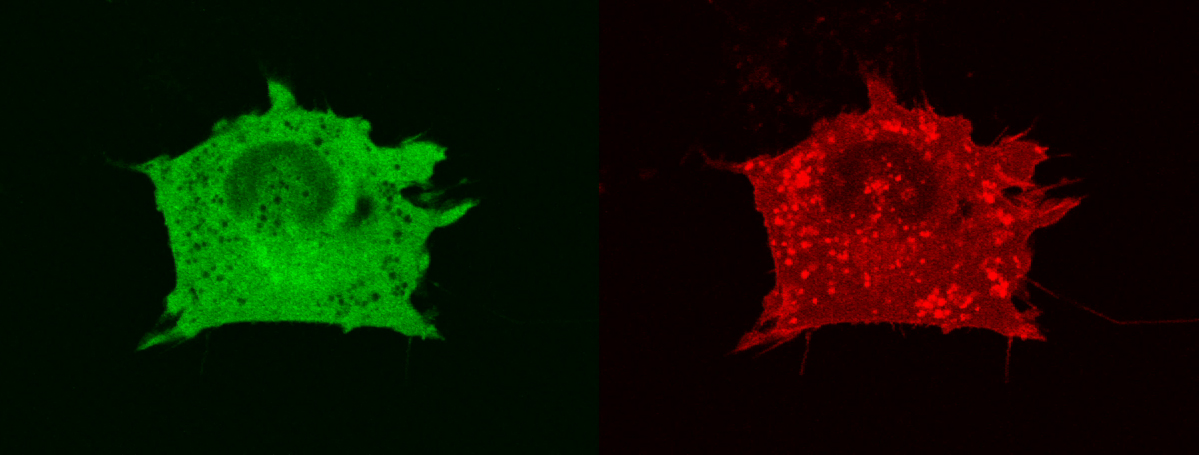
Movie 2 – MTE 4-14 cells transfected with Trop-2-mRFP1 and with PKCδ–EGFP. Cytoplasmic vescicles containing Trop-2-mRFP1 are useful landmarks for the localization of PKCδ–EGFP (empty cytoplasmic regions). No significant colocalization of Trop-2-mRFP1 and of PKCδ–EGFP was observed.
References
- Fornaro M, Dell’Arciprete R, Stella M, Bucci C, Nutini M, Capri MG, Alberti S. Cloning of the gene encoding TROP-2, a cell-surface glycoprotein expressed by human carcinomas. Int. J. Cancer 1995;62:610-8.
- Calabrese G, Crescenzi C, Morizio E, Palka G, Guerra E, Alberti S. Assignment of TACSTD1 (alias TROP1, M4S1) to human chromosome 2p21 and refinement of mapping of TACSTD2 (alias TROP2, M1S1) to human chromosome 1p32 by in situ hybridization. Cytogenet. Cell Genet. 2001;92(1-2):164-5.
- Ripani E, Sacchetti A, Corda D, Alberti S. The human Trop-2 is a tumor-associated calcium signal transducer. Int. J. Cancer 1998;76:671-676.
- El-Sewedy T, Fornaro M, Alberti S. Cloning of the mouse Trop2 gene – Conservation of a PIP2-binding sequence in the cytoplasmic domain of Trop-2. Int. J. Cancer 1998;75:324-331.
- Linnenbach AJ, Seng BA, Wu S, Robbins S, Scollon M, Pyrc JJ, Druck T, Huebner K. Retroposition in a family of carcinoma-associated antigen genes. Mol. Cell. Biol. 1993;13:1507-1515.
- Chong JM, Speicher DW. Determination of Disulfide Bond Assignments and N-Glycosylation Sites of the Human Gastrointestinal Carcinoma Antigen GA733-2 (CO17-1A, EGP, KS1-4, KSA, and Ep-CAM). J. Biol. Chem. 2001;276(8):5804-5813.
- Stensman H, Raghunath A, Larsson C. Autophosphorylation suppresses whereas kinase inhibition augments the translocation of protein kinase Calpha in response to diacylglycerol. J Biol Chem 2004;279(39):40576-83.
- Naquet P, Lepesant H, Luxembourg A, Brekelmans P, Devaux C, Pierres M. Establishment and characterization of mouse thymic epithelial cell lines. Thymus 1989;13(3-4):217-26.
- Soh JW, Lee EH, Prywes R, Weinstein IB. Novel roles of specific isoforms of protein kinase C in activation of the c-fos serum response element. Mol Cell Biol 1999;19(2):1313-24.
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 2004;22(12):1567-72.
- Sacchetti A, Subramaniam V, Jovin TM, Alberti S. Oligomerization of DsRed is required for the generation of a functional red fluorescent chromophore. FEBS lett. 2002;525(1-3):13-19.
- Sacchetti A, Alberti S. Protein tags enhance GFP folding in eukaryotic cells. Nat. Biotechnol. 1999;17:1046.

- Prof. Saverio Alberti
- Unità di Patologia Oncologica
- CeSI, Università “G. D’ Annunzio”
- Via Colle dell’ Ara
- 66013 Chieti Scalo (Chieti), Italy


