Home Research Centers Roswell Park Cancer Institute

The History of Flow Cytometry at Roswell Park Cancer Institute
by Carl and Sigie Stewart, and Paul Wallace
The Roswell Park Cancer Institute in Buffalo, New York, was the founded in 1896 as the nation’s first Cancer Research Institute. Originally named Roswell Park Memorial Institute, it became internationally recognized in the 1940’s and 50’s for its pioneering tissue culture work. RPMI 1640, one of the media formulations developed at Roswell Park, is still used by most lymphocyte biologists. The early history of flow cytometry at Roswell Park Cancer Institute is a history of individual efforts to gain knowledge of and experience with an emerging technology. Dr. Oskar Frankfurt of the Department of Experimental Therapeutics during the late 1970’s developed expertise in DNA content analysis, using an ICP22 – an early German-built flow cytometer. By 1978, the Department of Medical Oncology had purchased a FACS II cell sorter from Becton Dickinson which provided cell sorting service to a small group of investigators interested in tumor associated antigens.
Dr. Howard Osier joined the Department of Medical Oncology in 1980. Dr. Osier had a strong interest in expanding flow cytometry at Roswell Park to include clinical diagnosis using recently developed monoclonal antibodies from Becton Dickinson and Ortho Diagnostics. He purchased a FACS Analyzer (with a halogen lamp light source) and in collaboration with Dr. Tin Han developed single color protocols to analyze samples from patients with a syndrome subsequently identified as AIDS. Dr. Osier (and the FACSAnalyzer) departed Roswell Park in 1985 and further efforts to develop clinical analysis using flow cytometry were curtailed at Roswell Park until 1988 when Dr. Thomas Tomasi created the Laboratory of Flow Cytometry.
The Laboratory of Flow Cytometry at Roswell Park Cancer Institute began operation on November 1, 1988 under the direction of Dr. Carleton Stewart (Figure 1).
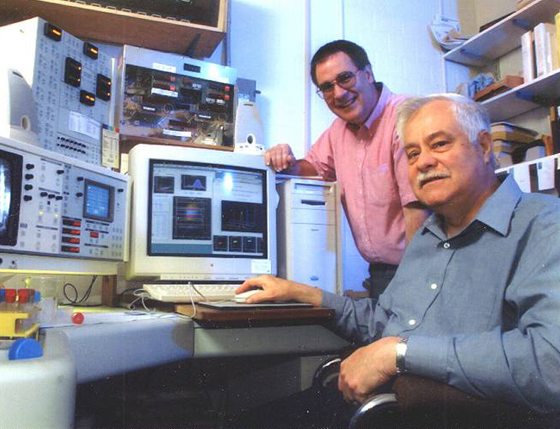
Figure 1: Carleton Stewart (seated), and David Sheedy
The laboratory’s vision was to provide state of the art multicolor flow cytometry services to both the Clinical and Research staff at the Institute. It was one of the first facilities to provide multicolor flow cytometry. Based on this early experience, the research focus of personnel was to develop and test strategies in multicolor immunophenotyping by flow cytometry for reliable processing of clinical and research specimens. The process included verification of reagent properties and quality, sample preparation, verification methods for instrument performance, automation of data acquisition and analysis and archiving data. To this day, every clinical file ever acquired by the laboratory since 1988 has been archived, representing one of the largest data bases in the world. By the end of 10 years, over 40,000 four-color patient files were being generated per year.
Flow Cytometers at RPCI Laboratory of Flow Cytometry http://www.rpciflow.org/facilities.html
The laboratory not only produced quality flow cytometry immunophenotyping data but also had, in cooperation with Becton Dickinson Immunocytometry Systems (BDIS), an instrumentation maintenance and development component. None of this development would have been possible without the support of Larry Duckett and John Cardot from BDIS and our own engineer, Ed Podniesinski (Figure 2).

Figure 2: BDIS collaboration with Roswell Park. From Left to right John Cardott, unknown, Carleton Stewart (seated), David Sheedy, Larry Duckett (seated), and Ed Podniesinski.
The first such effort was to develop a high-speed cell sorter (Figure 3).
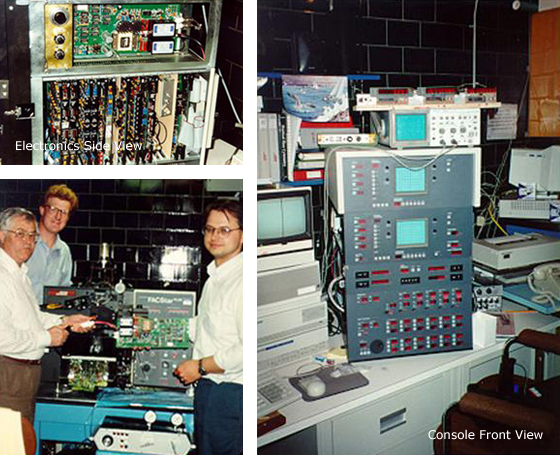
Figure 3: High speed FACSVantage sorter demonstrated at 1994 ISAC Lake Placid meeting by BDIS, capable of sorting 20,000 cells per second. Top left, electronics side view, right console front view, bottom left design team Carleton Stewart, Larry Duckett, and Ed Podniesinski.
To accomplish that, the FACStar Plus fluidics and electronics were modified to sort cells at an interrogation rate of 20,000 events/sec. After having successfully provided the proof of principal, BDIS built the first alpha Vantage that was shown at the 1994 ISAC meeting in Lake Placid, NY. During this period, the FACScan, a very popular easy to use instrument capable of three-color detection, was modified by us for four-color detection in a collaboration with BDIS. This then led to a totally new instrument, primarily for the research environment, the LSR. Today this instrument can be custom designed using several lasers, principally diode lasers for multicolor capabilities.
In 1996, development of a DNA instrument was underway at Roswell using a 355 nm HeCad laser in combination with a primary 488 nm laser (Figure 4).
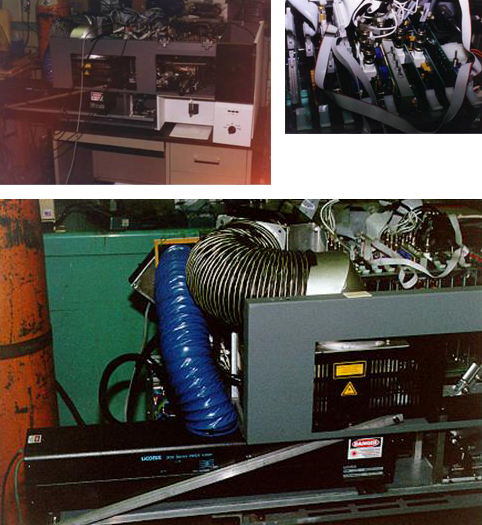
Figure 4: Single laser FACSsort modified at Roswell Park with two parallel beam lasers a 355 nm UV and primary 488 nm line. Delay line circuitry was developed to separate UV signal in time from 488 nm signals, BD later used this concept for the FACS Calibur.
Early in this instruments development a decision to use parallel instead of collinear beams was made because of concerns over spectral overlap. To accomplish this task, resultant interrogation data from the UV laser had to be stored electronically until the data off the 488 nm line was collected. Based on electronic designs used in ship radar, Ed developed a delay line circuit. This circuit was subsequently used by BDIS in the FACSCalibur.
The next period of instrument development has been the addition of infrared excitation and emission detection (Figure 5) using a 785 nm laser and an avalanche photon diode (APD) detector. (C.C. Stewart, M.L. Woodring, E. Podniesinski, B. Gray. Flow Cytometer in the Infrared: Inexpensive Modifications to a Commercial Instrument. Cytometry Part A 67A:104-111, 2005). This new ability extends the dynamic range of flow cytometry from the UV to the infrared. A fully operational instrument has been on line for five years.

Figure 5: IR Flow Cytometer (785 nm laser), with avalanche photon diode (APD) detector.
Only one model flow cytometry laboratory has existed at RPCI since 1988, and all flow services for the entire Institute are provided and maintained by this laboratory. The highly trained personnel (http://www.rpciflow.org/staff.html), nearly all of whom have at least five and some more than 25 years of flow experience, perform quality assurance on all instruments on a daily basis. Satellite facilities were established close to frequent users for their convenience but they are still under the maintenance and supervision of laboratory personnel. We firmly believe good data can only be acquired by well-maintained instruments with advice and council from the staff.
In June of 2003, Sigie and Carl retired six months after the new Director, Paul Wallace, had arrived so there could be some overlap. While continuing the original model in many ways, Paul has introduced new ideas and capabilities to the laboratory, most notable the responsibility for microscopic imaging. In 2007, the Facility was renamed the Department of Flow and Image Cytometry and acquired an Amnis ImageStream cytometer capable of capturing multiparameter images of cells in flow. The facility also provides confocal and kinetic microscopy services but retains its roots in Flow Cytometry. Today most of the cytometers have been upgraded with digital electronics and 5 and 6-color clinical flow cytometry has become the norm. The more adventurous researchers at the Institute are performing 10-color experiments on the facility’s LSRII and FACSAria and Ed Podniesinski has been experimenting with a breadboard multi-spectral flow cytometer using a 26 channel APD array (Figure 6).
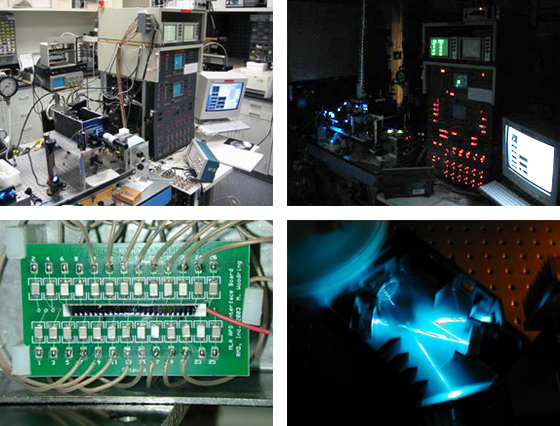
Figure 6: Multi-spectral flow cytometer using a 26 channel avalanch photon detector (APD). Top left and right, front view of breadboard cytometer; bottom right, Ocean Optics HR-2000 spectrometer; bottom left 26 channel APD array pictured here are 2 mm wide individual elements.
