Home Research Centers Max Planck Institut

History & Concepts of Martinsried Flow Cytometry Group
by Günter Valet
Introduction
Cytophotometry (1) and electronic cell counting (2, 3) had generated in a historical context already early on significant interest amongst biomedically oriented scientists. For electronic cell counting it took, however, a certain time until eminent clinical hematologists were convinced of the usefulness of electronic red and white blood cell counts as well as of platelet counts (4, 5, 6) in comparison to the long established counting chamber methods.
To speed up the acceptance process, Wallace Coulter as a globally thinking entrepreneur travelled around 1958/59 personally with a Coulter Model A counter in his luggage to various institutions in Europe that he considered of strategic interest. One of these institutions was the Max-Planck-Institute für Biochemie in Munich (MPI-Biochemie), headed by nobel laureate Adolf Butenandt, where he addressed Gerhard Ruhenstroth-Bauer, a hematology oriented scientist and director of the Department of Experimental Medicine. Ruhenstroth was interested, bought a Coulter A counter with a serial number around 550 and the particular interest to measure volume distribution curves of cells. This was possible because the signal amplitude of the counting pulses is proportional to cell volume. He considered this feature of the instrumentat of particular interest for the better characterisation of blood, leukaemia and cancer cells.
Thus Klaus and later Odila Zang, two young scientists of his laboratory, investigated volume distribution curves of various cell types (7) and recognized that Coulter cell volume distributions were right skewed for erythrocytes. This was considered of probably artefactual nature since from previous microscopic and electron microscopic evidence, symmetric Gaussian normal distribution curves were expected. Wallace Coulter, being primarily interested in the use of electronic blood cell counting for clinical purposes, was not enthusiastic about these unforeseen findings and considered legal action against the MPI-Biochemie for distributing non advantageous rumours about his counter.
Early instrument development
Ruhenstroth, remaining highly interested in the determination of cell volume distribution curves, circumvented potential anger with Coulter by asking Butenandt for an equivalent of 250.000 Euro (countervalue of 10 high speed ultracentrifuges at this time) to develop new instrumentation. Butenandt was hesitant but finally made these comparatively enormeous funds available. Jürgen Gutmann was hired as electronic engineer, an electronic workshop was equipped and the instrument building phase of the institute began (8 → Fig. 1, 9, 10, 11 → Fig. 2 left) in its former location in central Munich, close to the main railway station (Goethestrasse 33) from where the institute moved to the newly built Martinsried facilities in 1972.
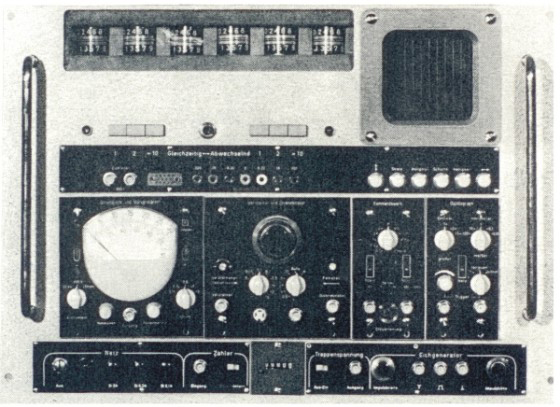
Fig. 1: Electrical impedance cell sizing unit, J. Gutmann, Diplomarbeit Fachbereich Elektrotechnik TU-München 1963
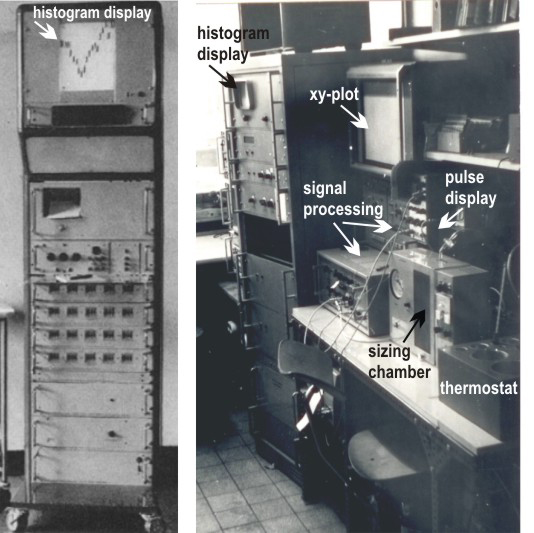
Fig. 2: Left: Signal processing & histogram display unit from: J. Gutmann, V. Kachel, R. Röttger, G. Ruhenstroth-Bauer, Naturwiss 55:130(1968) (reproduction with kind permission of © Springer-Verlag Berlin Heidelberg), Right: Metricell, Dec. 1971 (image: © Günter Valet)
Klaus & Odila Zang, Jürgen Gutmann, Mac Fulwyler (12) and others observed the right skew of Coulter volume distribution curves and considered it an artefact. Gutmann hypothesized that biconcave erythrocytes went through the sizing orifice in variable orientation like lengthwise, transverse or in intermediate position, causing variable displacement of electrical field lines leading to increased signals for transversely passing erythrocytes. Right skew in nucleated cell volume distribution curves was interpreted as representing in part size difference during cell cycle.
Metricell
Gutmann’s concept was not confirmed by the subsequent investigations initiated by Reinhard Thom, a clinician from Berlin (Klinikum Westend, Freie Universität). He modified a standard Coulter orifice by a hydrodynamically focused cell influx capillary in front of the sizing orifice while being a guest scientist at the MPI-Biochemie (13, 14, 15). The available sizing instrumentation Metricell as developed by Volker Kachel (16) (Fig.2 right) in continuation of Gutmann’s earlier work was used for the measurements.
The need for a photographic visualization of particles on their way through the orifice required an ultrashort flashlight (40nsec) and a special observation chamber (16 → Fig. 4a). This quartz chamber had been fabricated by Zeiss (Oberkochem) during a collaborative effort 1966/67 as a consequence of the development of the first optical flow cytometer in a modern sense by Kamentsky (17). In this cytometer, cell DNA was determined by optical absorption at 253.7 nm simultaneously with a light scatter measurement at 410.0 nm. The project idea was to measure additionally cell protein content at 280 nm as a simultaneous three parameter measurement. Zeiss had provided a tuneable monochromator with an HBO200 mercury arc lamp and a sophisticated microscope. The measurements were technically possible but it became clear that the observed signals were mostly light scatter signals that were not sufficiently specific to separate cell DNA and protein of unstained cells in flow. The project was abandoned but the quartz chamber, kindly donated by Zeiss, was essential for the extensive subsequent high speed photographic investigations of cells passing through electrical cell sizing orifices.
After detailed investigations, it was shown that cells traveling over the edges of the orifice entrance, as in the typical Coulter counter, passed through zones of increased electrical field strength, causing higher signals than for cells passing through the center of the orifice. Focusing particles on restricted pathways through the orifice provided the postulated symmetrical volume distribution curves for erythrocytes (14, 18) or monodisperse microbeads. Spielman & Goren (19) had equally observed a narrowing of Coulter volume distribution curves by hydrodynamic focusing around the same time but did not provide explanations for their observation.
Conclusion: The extensive experimental work connected with the proof of the hypothesis that the right skew of volume distribution curves in normal Coulter counters at unrestricted particle path through the sizing orifice was an artefact has led to the development of the cell sorter (12) as well as to fast imaging in flow (20) 4. Data analysis.
A major bottleneck in cytometric investigations concerned data display and data analysis. The initial hardware solution at MPI-Biochemie consisted of a set of around 20 relays having little weights attached to strings running over small step wheels at each relay. The wheels were advanced by the relays after a given number of counted cells within a given range of maximum pulse amplitudes as equivalent of cell size. This provided “negative histograms” (Fig. 2 left). The absolute cell count for each histogram channel was separately printed on paper. The use of an oscilloscope as histogram display and of an xy-plotter to permanently visualize the measured histograms (Fig. 2 right) improved record keeping but the quantitative information for histogram comparison and further analysis remained still printed on paper.
The increased resolution of hydrodynamically focused measurements of cell volume distribution curves led to the discovery of discrete erythrocyte populations by size during the first trimenon of life (21) in various mammalian organisms or in adults after x-irradiation (22) or strong bleeding. Depending on species, these populations showed in addition different hemoglobins, antigen expression or electrophoretic mobility as consequence of switched gene expressions patterns.
Having shown that the volume distribution curves had the potential to monitor gene activation patterns in the hematopoietic system, a more detailed data analysis was required to understand the sequence of events. The printed cell contents of the various channels of the volume distribution histograms were analysed in a first approximation on probability paper (21, 22) to obtain a model of the sequence of cell populations, thus concentrating the many initial histogram channel counts into a sequence of means, standard deviations and % contribution of the various cell populations over time.
Computers were at this time far too costly to purchase them for such an analysis. The move of the institute to Martinsried in 1972 provided, however, access to a Siemens 4004 main frame computer that had been purchased by the Max-Planck Gesellschaft for the equivalent of around 2 million Euros to advance the analysis of electron microscope and x-ray crystallography data. The computer had a core memory of around 1 Mbyte. From now on, cell volume distribution curves could be iteratively fitted by standard Gaussian normal distributions or by other functions with substantially more information and knowledge being extracted (23, 24) than by the visual inspection of histograms.
FLUVO-Metricell
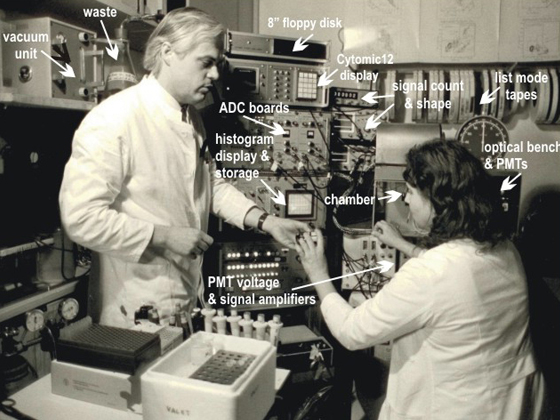
Fig. 3: Fluvo Metricell 1982, Günter Valet, Hanna Kahle, (reproduction with kind permission of © KNA Bild, Bonn)
With the introduction of fluorescence into flow cytometry by Wolfgang Göhde (25, 26) and into cell sorting in Len Herzenberg’s laboratory (27), flow cytometry gained access to an essentially unlimited number of specific molecular stains. It seemed important for medicine oriented cell biochemistry to develop into this direction. Phywe company (Göttingen) commercialized Göhde’s instrumentation but difficulties existed in purchasing only the optical part of the instrument since the intention at MPI-Biochemie was to use own electronics for data acquisition and a computer for data evaluation. A member of the Phywe board of directors happened to be at the same time senator of the Max-Planck-Gesellschaft. He arranged that the optical part of the first commercially produced Phywe ICP11 instrument (W Göhde personal communication) could be purchased in 1969.
Despite high interest, it took until 1977 to build the FLUVO-Metricell flow cytometer (28) (Fig. 3). It measured hydrodynamically focused electrical cell volume and two fluorescences. This instrument had hardware display units but was at the same time connected to a Perkin Elmer INTERDATA 7/32 computer with a 220 Mbyte hard disk drive and a 9-track tape unit for data collection. This provided essentially unlimited list mode storage capacity as well as significant list mode evaluation potential. The total unit was for years in advance of commercially available instrumentation. During the FLUVO-Metricell instrument development, cell volume studies as well as software developments were further advanced. The use of electrical sizing instead of light scatter as in laser instruments complicated the hardware but was considered essential in a cell biochemistry environment to be able to calculate relative substance concentrations in cells as well as relative molecule packing densities on cell surfaces.
The concentration on cell functions (functional flow cytometry, functional cytomics) as fast indicators of disease activity in patient cells was a main strategic goal at MPI-Biochemie. Stains for the flow cytometric determination of intracellular pH, Ca2+, free radicals, glutathione, proteases, esterases and phosphatases or for transmembrane and mitochondrial potentials in single viable cells were developed, using propidium iodide to monitor simultaneously the DNA of dead cells at UV or blue light excitation (for details refer to 29, 30). Initial efforts of a significant number of hospitals and research institutions using the standard mercury arc lamp equipped Phywe ICP11 flow cytometer, mostly in Germany, concerned preferentially clinical DNA analysis in malignant disease. The Los Alamos and Livermore laboratories with their laser flow cytometers and cell sorters were substantially centered on cell cycle and chromosome research, the Sloane Kettering group on DNA conformation in the cell cycle of normal and abnormal cells.
Mildred-Scheel Laboratory for Cancer Cell Research (1981-1989)

Fig. 4: Opening of the Mildred-Scheel Laboratory for Cancer Cell Research at Max-Planck Institut für Biochemie, Martinsried on July 15, 1981, Dr. Mildred Scheel (President Deutsche Krebshilfe), Prof. Peter Hans Hofschneider (Institute Director) (reproduction with kind permission of © MPG-Pressestelle, Munich)
Dr. Mildred Scheel, the wife of former German Bundespräsident Walter Scheel, had founded the Deutsche Krebshilfe as well as the Mildred-Scheel Foundation to provide better care for cancer patients and to advance cancer research in Germany. Following significant funding of clinical institutions, certain criticisms emerged that not enough was done for the research sector. This prompted the Mildred-Scheel Foundation together with the Max-Planck Gesellschaft zur Förderung der Wissenschaften to internationally announce a 5 year research project in the area of medicine oriented basic cancer research. Günter Valet applied as did more than 50 other scientists and was finally appointed head of the independent Mildred-Scheel Laboratory for Cancer Cell Research (Fig. 4) after a thorough two year selection process by a search committee, composed of around 20 scientists coming about equally from Deutsche Krebshilfe, the Max-Planck-Gesellschaft as well as from several universities and large cancer research institutions in Germany.
The submitted project proposal aimed at the simultaneous multiparameter analysis of single cells by flow cytometry as a sensitive approach for the detection and molecular characterization of cancer cells in patients as well as at the development of suitable benchtop instrumentation for this purpose. The Mildred-Scheel Foundation provided the equivalent of 1.5 Mio Euro for salaries while the Max-Planck Gesellschaft contributed the laboratories and running costs in the order of 0.5 Mio Euro. Dr.Scheel as former radiologist, followed the project with close attention but died in 1985 of cancer.
Martinsried Flow Cytometry: Intracellular pH & Esterase activity in rat bone marrow cells
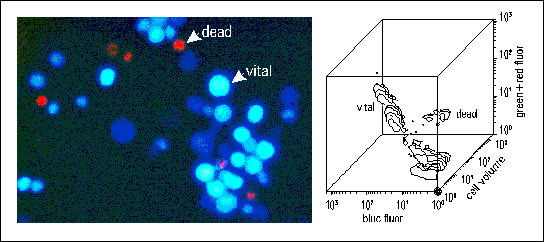
Fig. 5: Cell function in cytomics: left: intracellular pH & esterase staining (ADB/DCH) of viable rat bone marrow cells (blue) and of the DNA in dead cells (PI) (red). right: contour line display of the flow cytometric measurement of around 6.000 cells of the stained cell preparation from the left panel.
Major focus points of the scientific project work concerned the:
- Establishment of sensitive cell function assays for cancer cell detection by flow cytometry (Fig. 5)
- Development of individualized cytostatic drug assays for patient cells
- Development of data pattern analysis for knowledge extraction from complex multiparameter data
- Application of this new potential in patient studies in collaboration with a variety of clinical institutions
- Development of benchtop instrumentation (FLUVO-Metricell II, Cytomic123, 31, 32, 33, 34) by Volker Kachel
- For further details of the cell biochemistry group see literature references 1981-1990.
After 5 years, the project was prolonged for another 3 years until 1989, amounting to a total funding of around 3.2 Mio Euro. It was at this time the largest project the foundation had ever funded. About 10 FLUVO-Metricell II instruments, data recording or display modules were produced by the technical group and sold to scientific institutions between 1984-1989 with reinvestment of the income into further research. The reasoning for the termination of the project in 1989 was that flow cytometry had become a routine technology with no remaining research requirements in the earlier context.
Cell Biochemistry Group
Following the termination of the Mildred-Scheel Laboratory project, the cell biochemistry part of the Martinsried flow cytometry group remained as Cell Biochemistry Group scientifically independent and continued the work with funds from European research projects, through Sonderforschungsbereiche of the Deutsche Forschungsgemeinschaft (DFG) and basic funding by the Max-Planck-Gesellschaft.
Given the fast technological progress, commercial instrumentation such as the PASIII cell analysis and closed piezo sorter system (Partec, Münster, Germany) met increasingly the needs of basic cell biochemistry research and did not require further in-house technological development. Likewise, personal computers provided enough computing power for cytometric list mode data analysis. Decreasing funds and number of collaborators in combination with the accumulation of increasing multiparametric data sets in many clinical environments produced a gradual shift from the production of own data to processing other groups data with the advantage that it would have been experimentally not easily possible by own investigations to generate the amount of experimental & clinical data required for the gradually developed concepts.
With decreasing funding and a comparatively low interest for flow cytometric cell biochemistry at the national level (see frequency of terms like Zytomik or Humanzytomprojekt in the Internet), the concentration onto one of the intellectual core competences being knowledge extraction from complex multiparameter data became increasingly important for the successful continuation of the work.
Essential transit points in this effort over time were:
- The automated diagnosis from flow cytometric list mode data (1987)
- The view that cytometry and later cytomics constitute a biomedical key discipline where the explicit analysis of the heterogeneity of cell systems in form of system cytometry (1997) represents a comparatively efficient top-down strategy for the systematic resolution of the cellular and molecular biocomplexity of higher organisms. One of the important advantages being that complex disease mechanisms can be investigated without necessity for extensive a-priori knowledge and molecular pathway modeling.
- The individual patient disease course prediction by SMDC (predictive medicine by SMDC) (2000) (Fig. 6)
- The definition of cell systems as cytomes (2001) in combination with
- The introduction and redefinition of the plant science term cytomics for cell biochemical purposes
- The predictive medicine by cytomics concept (2001) as well as
- The elaboration of concepts for a human cytome project (2004) and for a periodic system of cells (2005)
Martinsried Flow Cytometry: Data pattern analysis of patients at risk for myocardial infarction
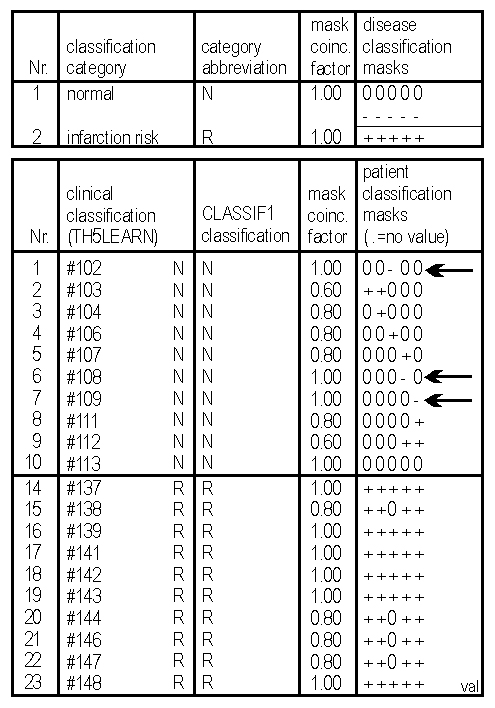
Fig. 6: CLASSIF1 data pattern analysis: Discriminatory disease classification masks (top of rightmost column) consisting in this case of the 5 most discriminatory out of 44 measured thrombocyte parameters, permit to correctly distinguish between risk and non-risk patients for myocardial infarction from molecular properties of peripheral blood thrombocytes. The CLASSIF1 algorithm (CLASSIF1 classification column) correctly recognizes the two types of patients (clinical classification column) from the analysed flow cytometric data (details, 35)
Overall Impact (1960-2006)
The Martinsried flow cytometry group in its cell biochemistry and technology branches has contributed to several developments and concepts that have significantly shaped the cytometry field over time:
- The early instrument development (see above) terminated the long going controversy about the right skewedness of cell volume distribution curves by electrical sizing. Besides hydrodynamic focusing, it provided early computerized list mode storage as an important prerequisite for the efficient extraction of information and knowledge from multiparameter flow cytometry measurements.
- The development of flow cytometric cell function assays enabled the molecular evaluation of disease states as well predictions about the future disease progress in patients. The flow cytometric cell function area has advanced on its own into a steadily growing field in medicine but also in the pharmaceutical industry in the form of high-throughput and high-content assays for drug discovery both by flow cytometry and image cytometry.
- The development of data pattern analysis (data sieving) by the CLASSIF1 algorithm permits the exhaustive extraction of information from flow cytometric list mode files or other multiparameter data, thus providing standardized multiparameter data analysis and knowledge extraction.
- Data pattern analysis enabled amongst others the development of the concepts of individual patient disease course prediction by SMDC (standardized multiparameter data classification) and system cytometry as well as the cytome & cytomics definitions (Omics-glossary (2001)). Based on the predictive medicine by cytomics concept, the concepts of a human cytome project and of a periodic system of cells by cytomics were developed.
External feedback
The experimental and conceptual work of the Martinsried Cell Biochemistry Group has led to diverse forms of external interest such as the election into leading positions of international scientific societies, membership in various editorial boards, associate editor & editor of the “cytomics” editorial column in Cytometry A, invitation to more than 20 review articles in the cytomics area since 2001 & more than 200 invitations for presentations of the groups cell biochemistry work at scientific meetings or in many institutions worldwide since 1981, furthermore to awards, membership in scientific advisory boards and collaboration with the pharmaceutical industry in the area of predictive medicine by cytomics ( → personalized medicine, individualized medicine). The presently widespread international interest is also reflected by inclusion of various definitions and concepts into on-line glossaries like Omes & Omics or encyclopedias like Wikipedia (cell biochemistry, cytomics, human cytome project) or in the ranking of the groups Internet pages by search engines like Google (date: position/total hits):
- Cell Biochemistry
- 14.04.07: 4/42.4mio
- “Cell Biochemistry”
- 18.11.04: 5/75.400
- 14.04.07: 3/161.000
- Predictive Medicine
- 02.08.01: 32/43.000
- 26.02.03: 5/75.400
- 14.04.07: 4/1.69mio
- “Predictive Medicine”
- 14.04.07: 4/88.100
- Cytome(s)
- 02.08.01: --/-- (0/0)
- 10.08.01: --/-- (1/3)
- 14.04.07: 3/88.900 (1/337)
- Cytomics
- 02.08.01: --/81
- 05.04.02: 1/150
- 07.09.05: 6/9.730
- 14.04.07: 3/40.600
- Human Cytome Project
- 12.12.03: --/3
- 16.12.03: 3/10
- 06.04.05: 1/139
- 14.04.07: 3/14.000
Outlook
The fields of cytometry and cytomics have been fascinating over many years by the collaboration of scientists who were interested in trans-disciplinary (cross-disciplinary) concepts and their potential to provide entirely new insights into cellular biomechanism. Flow cytometry and image cytometry instrument developers were initially (1965-1985) rather competing against each other for supremacy in the sensitive detection of cancer cells but the efforts have become more and more collaborative and mutually complementing in recent years. Flow cytometry as a fluorescence oriented technology has substantially enhanced the development of new stains, proving equally useful for molecular biology as for single cell oriented image cytometry like in confocal or laser scanning microscopy.
The cytometric field continues to be fascinating through its potential to unfold the organismal biocomplexity (organismic biocomplexity) top-down by single cell molecular analysis in-situ that is with all molecules in place, lending itself to the reverse engineering of the assembled molecular machinery as well as to the investigation of the natural heterogeneity of cells in tissues and in complex disease mechanisms. This seems particularly promising since the knowledge of the entire set of biomolecules as derived at the genome level does by itself so far not provide enough information for the molecular reassembly in form of living cells, tissues, organs and organisms. The fascination for the cell biochemistry & cytomics fields will therefore in all likelihood not only continue but further increase, seen the overall potential and challenges of this approach.
Literature References
- T Caspersson. Cell growth and cell function, a cytochemical study. Norton, New York (1950).
- WH Coulter. Means for counting particles suspended in a fluid. US patent 2,656,508 priority Aug 27, 1949.
- WH Coulter. High speed automatic blood cell counter and cell size analyzer. Proc Natl Electronics Conf 1956, 12: 1034-1042.
- G Brecher. Evaluation of electronic red blood cell counter. Am J Clin Pathol 26:1439-1449 (1956).
- WJ Richar, ES Breakell. Evaluation of an electronic particle counter for the counting of white blood cells. Am J Clin Pathol 31:384-393 (1959)
- CR Sipe, EP Cronkite. Studies on application of Coulter electronic counter in enumeration of platelets. Ann NY Acad Sci 99:262-270 (1962)
- G Ruhenstroth-Bauer, D Zang. Automatische Zählmethoden: Das Coulter’sche Partikelzählgerät. Blut 6:446-462 (1960)
- J Gutmann. Diplomarbeit Fachbereich Elektrotechnik, TU-München 1963
- O Zang. Untersuchungen über die Volumenbestimmung von Zellen nach dem Widerstandsmeßprinzip von Coulter. Dissertation Medizinische Fakultät, LMU München 1965
- KD Zang. Automatische Zählung und Volumenbestimmung von Zellen auf elektronischem Wege. Deutsche Med Wschr 84:1710-1716 (1964).
- J Gutmann, V Kachel, R Röttger, G Ruhenstroth-Bauer. Numerical recording and processing of electrical measurements Naturwiss 55:130 (1968)
- MJ Fulwylwer. Electronic separation of biological cells by volume. Science 1965, 150: 910-911.
- R Thom. Anordnung zur Gewinnung von Gröβen, die den Mengen von in der Untersuchungsflüssigkeit enthaltenen Teilchen verschiedenen Volumens entsprechen. Patent DE1806512, priority Nov 02, 1968.
- R Thom, A Hampe, G Sauerbrey. Electronic cell volume determinations and its sources of error. Zsch Ges Exp Med 151:331-349 (1969)
- R Thom, V Kachel. Advances for electronic determination of size of erythrocytes. Blut 21:48-49 (1970)
- V Kachel. Basic principles of electrical sizing and their realization in the new instrument “Metricell”. J Histochem Cytochem 24:211-230 (1976)
- LA Kamentsky, H Derman, MR Melamed. Spectrophotometer: New instrument for ultrarapid cell analysis. Science 1965, 150: 630-631.
- V Kachel, H Metzger, G Ruhenstroth-Bauer. Der Einfluβ der Partikeldurchtrittsbahn auf die Volumenverteilungskurven nach dem Coulter Verfahren. Zschr Ges Exp Med 153:331-347 (1970)
- L Spielman, SL Goren. Improving resolution in Coulter counting by hydrodynamic focusing. J Coll Interf Sci 26:175-182 (1968)
- V Kachel, G Benker, L Lichtnau, G Valet, E Glossner. Fast imaging in flow: A means of combining flow cytomety and image analysis. J Histochem Cytochem 27: 335-341 (1979)
- G Valet, H Metzger, V Kachel, G Ruhenstroth-Bauer. Die Volumenverteilungskurven von Rattenerythrozyten nach Röntgenganzkörperbestrahlung. Blut 24: 274-282 (1972)
- G Valet, H Metzger, V Kachel, G Ruhenstroth-Bauer. Der Nachweis verschiedener Erythrozytenpopulationen bei der Ratte. Blut 24: 42-53 (1972)
- G Ruhenstroth-Bauer, G Valet, V Kachel, N Boss. Die elektrische Volumenmessung von Blutzellen bei der Erythropoese, bei Rauchern, Herzinfarkt- und Leukämiepatienten, sowie von Leberzellkernen. Naturwiss 61: 260-266 (1974)
- G Valet, W Opferkuch. Mechanism of complement induced cell lysis. Demonstration of a three step mechanism of EAC1-8 cell lysis by C9, and of a non osmotic swelling of erythrocytes. J Immunol 115: 1028-1033 (1975)
- W Göhde Automatisches Meβ- und Zählgerät für die Teilchen einer Dispersion. Patent DE1815352, priority Dec 18, 1968.
- W Dittrich, W Göhde. Impulsfluorimetrie bei Einzelzellen in Suspensionen. Z. Naturf 24b:360-361 (1969).
- HR Hulett, WA Bonner, J Barrett, LA Herzenberg Cell Sorting: Automated separation of mammalian cells as a function of intracellular fluorescence. Science 166:747-749 (1969).
- V Kachel, E Glossner, E Kordwig, G Ruhenstroth-Bauer. Fluvo-metricell, a combined cell volume and cell fluorescence analyzer. J Histochem Cytochem 25:804-812 (1977)
- G Valet. Die Durchflusszytometrie im Wandel der Forschungskonzepte. In: Angewandte Durchflusszytometrie. Eds: U Sack, A Tarnok, G Rothe. Karger Verlag, Basel (2007) p.1-26
- G Valet. Past and present concepts in flow cytometry: A European perspective. JBRHA 17:213-222(2003)
- V Kachel, H Schneider, K Schedler. A new flow cytometric pulse height analyzer offering microprocessor controlled data acquisition and statistical analysis. Cytometry 1:175-192 (1980).
- V Kachel, K Schedler, H Schneider, L Haak. “Cytomic” data system modules – modern electronic devices for flow cytometric data presentation and handling. Cytometry 5:299-303 (1984).
- V Kachel, H Schneider. On-Line three-parameter data uptake, analysis and display device for flow cytometry and other applications. Cytometry 7:25-40 (1986).
- V Kachel, R Messerschmidt, P Hummel. Eight-parameter PC-AT based flow cytometric data system. Cytometry 11:805-812 (1990)
- G Valet, M Valet, D Tschöpe, H Gabriel, G Rothe, W Kellermann, H Kahle. White cell and thrombocyte disorders: Standardized, self-learning flow cytometric list mode data classification with the CLASSIF1 program system. Ann NY Acad Sci 677: 233-251 (1993)
