Home Cytometry History General Histories Leiflight

Particle Technologies, Inc and the history of instrument development
by Robert Auer

I met Mack J. Fulwyler, PhD, the President of PTI, and from him heard for the first time the story of flow cytometry and sorting, a technology based on his PhD Thesis project. I met Mike Trump, PTI’s Chief Engineer, and John Glascow, the other engineer on staff. I met Jim Corell and Bill Hatcher, two refugees from Sandia. Jim was a physicist who could be best described as “inventor in residence” and Bill was an organic chemist who was making microspheres out of plastic one at a time at the rate of thousands per second using a technology that Mack had invented. I met Bill Schaff and Marilyn Campbell, the biochemist and medical technologist in the group. We drove to another building about a mile away where PTI’s shop was located. There I met Tom Tucker, Mack’s ex-brother in law, Dave and Diane Best, and Bill Aldridge. Tom and Dave were extraordinary machinists. Diane was PTI’s purchasing department. Bill did everything from finishing materials, to assembly, to running errands. I learned that PTI was a subsidiary of a “big” medical instrument company and that PTI’s charter was to spin the flow cytometry and microsphere technologies out of the H division of Los Alamos Scientific Laboratories in order to commercialize them. I found it to be a dynamic, exciting group working in a new technology of incredible potential. In September of 1973, I joined PTI (Particle Technology Inc.) in Los Alamos, New Mexico.
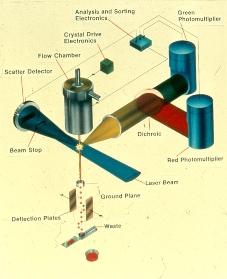
TPS schematic
When I arrived at PTI, there were four prototype instruments. The first prototype instrument was the SDS-1 (Super Duper Sorter); a sorter which measured two colors of fluorescence, forward light scatter, and electronic cell volume. It used a Spectra Physics 164, 5 watt argon laser for excitation. All data acquisition and sort decisions were via a PDP-8 computer with graphic displays on a Tektronics 4010 display. Programs and data were stored on Tridata cartridge tape. All of the programs were written in PAL8. This instrument was large, it occupied three six-foot high instrument racks. The second prototype instrument was a two color fluorescence plus forward scatter analyzer with a Spectra Physics 162, 15 milliwatt argon ion laser for excitation that also used a PDP-8 based data acquisition system. The third prototype was the SPA-1, a single color fluorescence only analyzer with a Spectra Physics 162, 15 milliwatt argon ion laser with a hardwire pulse height analyzer. Finally, John Glascow was in the process of building the TPA-1. This was a single color fluorescence plus forward light scatter analyzer with a Spectra Physics 162, 15 milliwatt argon ion laser with a hardwired pulse height analyzer. The TPA-1 was to be transferred to our parent company for commercialization. My task was to convert the TPA-1 into a sorter, the TPS-1. Soon after I arrived, John Glascow made the decision to leave the company so both the TPA-1 and the TPS-1 projects became my responsibility. In September and October of 1973, I worked on simplified flow chamber designs that would permit optical measurements with a 15 milliwatt laser and droplet formation for sorting.

The TPS
In November of 1973, I met for the first time Wallace H. Coulter, the principal shareholder of our parent Company, Coulter Electronics. Wallace; Walt Hogg, Coulter Electronics’ Chief Engineer; and Bob Klein, Coulter Electronics’ Director of Product Engineering, came to Los Alamos from Florida on their way to the Engineering Foundation Meeting on Analytical Cytology in Asilomar. There was much discussion about what was the right product for the market. Mack wanted to commercialize a large instrument like the SDS-1 from Los Alamos while Bob Klein wanted the smaller TPA and TPS products that would be made in Miami. After several days of reviews of our projects, Mack, Mike, Bill, Jim, and I joined them to travel to Asilomar. In Asilomar, I learned for the first time how broad the field of flow cytometry already was. On the way back to Los Alamos after the meeting, I paid a visit to Dick Sweet at Len Herzenberg’s lab at Stanford. Dick showed me his sorter that used in-the-jet sensing. This seemed like a simple solution to the flow chamber problem but required larger lasers for high sensitivity.
I was given the goal of having both the TPA-1 and TPS-1 prototypes at the FASEB meeting in Atlantic City the next April (1974). After a busy winter, that goal was realized. This was also the meeting at which BD also showed their first FACS instrument. After the show the TPA and TPS prototypes went to Florida where they were to be engineered for production. This became a considerable challenge because the engineers in Florida did not understand the technology. By the end of 1974, the redesign of the TPA-1 for production had not yet been accomplished. This product transfer problem lead to considerable disagreement and bad feelings between the team in Los Alamos and the larger engineering group in Florida. It was finally decided that I would undertake the re-engineering of the TPS-1 in Los Alamos and deliver a more refined prototype to Florida. This effort continued into the summer of 1975 when we delivered four redesigned TPS-1 prototypes to Florida. One of those prototypes went to Jerry Hudson’s lab at the Miami VA Hospital and another went to the Wheeless/Horan lab at the University of Rochester. As part of the TPS-1 project, I designed a scatter sensor that used a Fourier lens to reduce sensitivity to alignment and whose response was monotonic with particle size, this became my first patent.
New ideas and invention was an important part of the culture at PTI. This was led by Mack; he was always coming out of his office and saying, “think about this” and he would describe a new idea. We had weekly meetings in Mack’s office where, on a rotating basis, each staff member had to present a new idea and everyone else got to critique it. Some of these ideas turned into projects and patents; for example, an automated live cell/ dead cell enumeration system based on trypan blue exclusion detected by forward scatter and extinction, and “diagnosis on a microsphere.” This was Mack’s idea for using microsphere substrates to hold antibodies and target antigens as targets to diagnosis diseases. The tests could be multiplexed using microsphere size, fluorescence color and intensity as encoding. His patent on this was blocked in the US by a similar patent from Technicon but it issued in the UK.

The EPICS II
Based on input from potential customers, Mack continued to push to be able to build and sell the larger sorter systems. Early in 1975, PTI was awarded a contract by NIH to build and deliver a large multiparameter sorter to Dr. Chet Herman at NCI. Another engineer, Vaughn Rheems, was hired to work with Jim Corell to convert the SDS-1 prototype into a saleable system. This system, the EPICS II (Electronically Programmable Individual Cell Sorter), was shipped in July of 1975. The EPICS II generated considerable interest and there were several more orders in the works. Late in July of 1975, Mack wrote to Wallace asking permission to sell more EPICS II systems. Bob Klein argued against permission being granted. In early November, Mack went to Florida to plead his case. Because they could not reach agreement, Mack decided to terminate his relationship with Coulter. On November 14, 1975 it was announced to the employees that PTI would terminate operations on March 1, 1976. I was asked to move to Florida and manage the TPS-1 project and I did so in late January of 1976. We also moved the particle manufacturing technology. About a half dozen employees made the move to Florida. After a year at the Jovan lab in Germany in order to clear the non-competition clause in his contract, Mack went to work for BD.
The marketing group in Florida decided to skip the TPA-1 and go directly to the TPS-1. When I arrived in Florida, the TPS-1 was not yet in production. It took the better part of 1976 to get the first instruments out the door. At the same time the TPS-1 began to evolve, a second PMT was added to permit two color fluorescence measurement. It became clear that the 15 milliwatt laser was too low a power level for many applications when used with a sense-in-the-jet flow chamber, so an adaptation to a larger water cooled laser was designed. The factory was having trouble adapting to an evolving product that was being produced in small volume, so in early 1979 a dedicated manufacturing group was form that was closely coupled to the engineering team. I now had responsibility for manufacturing as well as engineering. This group became the EPICS Division of the Coulter Corporation and I was named the General Manager. Coulter Immunology was formed as a sister division to focus on monoclonal antibody reagents.
During this period it became obvious that the number of parameters that needed to be measured per cell was increasing. In order to accomplish this, the MDADS (Multiparameter Data Acquisition and Display System) was designed. This system contained two microprocessors; one to acquire the data and one to analyze and display it. This system could acquire eight parameters per event. The sensor system was also redesigned to permit a straight in coupling of the laser light into the beam shaping optics. In late 1979, the MDADS and the new sensor were introduced as the EPICS V. By 1981, the EPICS V had evolved to include three fluorescence channels. dual laser excitation configurations, and the measurement of light scatter at 90 degrees. In order to implement spatially separate dual laser detection, the Gated Amp technology was developed. This technology used temporal gating to detect the signals from each laser and delay lines to make the signals from the two lasers aligned in time for the data acquisition system. The EASY data analysis software package was developed first on a PDP-11 based graphics computer and then on a PC.

The EPICS C
In 1982, we introduced the EPICS C, a clinical cell sorter. In this instrument all operational settings were made via the onboard microprocessor. Protocols which included all settings could be created by the user and stored under a unique name. These protocols could then be called into the instrument which would reset the instrument to the previously defined operation state. This assured repeatable operation on a day to day basis. The ability to create and edit protocols was password protected. The EPICS C used a single water cooled argon ion laser and measured forward and side scatter along with two colors of fluorescence. There was an optional closed flow chamber with a biohazard containment waste system so that it could be operated as an analyzer.
By 1984, the EPICS V had evolved into the EPICS 750 series. This was a very large sorter system that had up to two gas ion lasers plus a dye laser. The measurement of forward and side scatter plus three colors of fluorescence was now the standard. Also in 1984, the role of the EPICS Division was expanded to include Sales, Service, and Customer support.

The PROFILE touch screen interface and graphics pad
In 1986, we introduced the EPICS PROFILE, a bench top clinical analyzer. It originally was introduced with either air cooled or water cooled argon ion lasers, but quickly the air cooled, 25 mw at 488nm configuration become standard. The flow chamber in the PROFILE utilized a lens and a mirror bonded to the quartz chamber which increased the amount of light collected. The instrument used a touch screen interface and a graphics pad as operator inputs. It was protocol driven and allowed chained protocols in a panel to pass information. The sample was presented by the operator to an aspiration probe and the touch bar was pressed to start a cycle. The instrument was designed for the standardized, repeatable measurements required of a diagnostic instrument.

The Q-PREP
During the development of the PROFILE, it became very clear that sample prep was a major hurdle to making flow cytometry a standard clinical technique. The standard process to isolate leukocytes from whole blood was a ficol gradient and centrifugation. This technique was time consuming, expensive, and very operator dependant. An alternative had to be found. The rapid removal of red cells via various chemical means was in the bag of tricks of our hematology coworkers at Coulter Diagnostics. They were constantly trying new ideas to improve their techniques. One technique that they investigated was a fast acid lysis with a base neutralization to stop the reaction. When done properly it appeared to have minimal effect on the leukocytes. It actually failed their testing because it produced red cell ghosts that interfered with electronic cell volume measurements, but the ghosts were not a problem with forward light scatter making it a perfect technique for flow cytometry. The reagent addition timing was critical. What was required was automation. What resulted was a synergistic combination of reagents and automation that changed sample prep from an hour long process that was very operator dependant to a 45 second process that was absolutely repeatable. In 1987 we released the Q-PREP sample preparation system.

Robert Auer answers: “How was the Q-PREP developed?”
Can’t view the videos? You may need a plugin or update. Click for suggested downloads
After our experience in transitioning from water cooled lasers to air cooled lasers in the PROFILE, we wanted to explore a similar paradigm change for the sorter instruments. The EPICS ELITE, released in 1988, was the result. This desk sized sorter system could easily be configured with multiple water cooled and/or air cooled lasers. It could use either sense-in-the-jet flow cells or closed flow chambers with high sensitivity optics. It used time domain gating technology to facilitate multiple laser sense points. A video camera was used to view the laser sense and break off points. The data acquisition and display was via an industry standard PC.

The ELITE CONSOLE
Also in 1988, the responsibility for the field sales and service operations was removed from the EPICS Division and given to the Diagnostics selling organization. This resulted in a reduced focus on the research cytometry marketplace. Engineering and manufacturing moved together into a new, two building facility.
In 1989 work began on a new clinical analyzer system to replace the PROFILE. The goal of this system was to eliminate analog fluorescence compensation and log amplifiers, both of which limited the measurement reproducibility because each such channel had its own personality. Going to an all digital system was contemplated but available fast ADCs were large and expensive and did not have sufficient dynamic range. It was determined that a 20 bit linear dynamic range was required to eliminate the need for log amps. In order to accomplish this, a hybrid analog/digital system was developed. The analog portion of the system provides dc restoration and adjustable gain to the signal from the transducer. The signal is then delayed for five microseconds to allow the trigger input to be activated ahead of the signal processing so that the whole signal can be processed. The delayed signal in each channel is applied in parallel to a gated wide dynamic range analog peak sense and hold and a gated wide dynamic range analog integrator. These two circuits produces dc values equal to the peak and area of the pulse respectively. Each dc result is passed through two paths; the first has unity gain and the second a gain of 32 (5 bits). The output of the high gain channel is compared to a slow moving ramp waveform that varied between 80% and 100% of the nominal linear range of the channel. If the amplitude of the output of the high gain stage is below the ramp value, then the signal from the high gain stage is captured for digitization. If the amplitude of the output of the high gain stage is above the ramp value, then the signal from the unity gain stage is captured for digitization. Since the occurrence of an event was asynchronous to the slow moving ramp, the decision point changed randomly. This serves to dither out any errors between the two gain channels. The captured dc outputs of all of the signal process channels are multiplexed to a single, very high linearity 16 bit ADC for digitization. Also captured is whether the dc value is from the high or unity gain channel. This information is used to route the high 15 bits of the ADC result to either the low 15 bits or the high 15 bits of a 20 bit word if the high gain or low gain channels are used respectively. In either case the remaining 5 bits are set to zero. The 20 bit results are then used for compensation calculations and the result can be converted to log data via a look up table. This new method of data acquisition was awarded US patent 5,367,474.
In addition to this new method of data acquisition, the new instrument has a much smaller table top foot print than the PROFILE. The air pressure and vacuum pump as well as all power supplies are incorporated into a floor mounted service module. The service module also holds the waste tank. The flow chamber retained the integral lens, but the mirror was eliminated. The mirror was found to contribute as much increased noise as increased signal, so the net gain was zero. The system acquires forward and ninety scatter plus four colors of fluorescence using a single 20 mw, air cooled argon laser. The system has a single tube manual feed station and an optional 32 tube carousel loader. The tubes in the loader could be directly identified via barcodes. System operation is via an external Personal Computer. The XL and XL/MCL were launched in late 1992 and began shipping in early 1993. That year the Multi-Q-Prep was also launched as a companion product. It serially performed the Q-Prep process on thirty two samples in the same carousel format as the XL/MCL (multi-tube carousel loader).
In 1993, the Coulter Corporation with the “help” of Anderson Consulting reorganized from a divisional business oriented structure to a functional structure. The tight coupling between cytometry instrument engineering and manufacturing was broken. Cytometry manufacturing joined the hematology manufacturing operation and co-located with them. Cytometry engineering became the “Research Products Platform” and moved with the hematology engineering groups to a new Corporate Campus. I became Platform Director of the Research Products Platform, which was essentially cytometry since both of the Clinical Products Platforms (large and small) were totally focused on hematology. The Coulter Corporation formed an Operating Committee of senior managers to meet on a monthly basis for peer review and discussion of the performance of the businesses; I was appointed a member of that group.
The software for the XL continued to evolve as the result of input from our customers and the efforts of our reagents and applications development teams. The system has the ability to run complex panels that contained a series of protocols incorporating data from multiple sample tubes. Locked Panels and DLLs (dynamic linked libraries) were added to perform automated analysis. QC and sample results are databased and can be interfaced to a laboratory information management system. TETRAOne, RETICOne, and STEMOne, which included reagent kits and DLLS, were released. We replaced the Multi-Q-Prep, which was designed by an external vendor and had reliability problems, with the much more robust TQ-Prep system.
In late 1994, we began discussions to acquire Immunotech in Marseille, France. We saw this as a source of a much needed research antibody pipeline and a site to pursue diagnostics for use outside of the US. The negotiations were not going particularly well until Joe Coulter Jr. became actively involved. He personally closed the deal in early 1995. By the end of 1995, Joe had succumbed to prostate cancer. Immunotech became part of my organization as did the US cytometry reagents group. As soon as we got together with the team at Immunotech, we started working on bead based multiplexed assays. They had an active EIA/RIA immunoassay business and we had the technology for making and dying bead substrates and for the instrumentation. Together we had the whole package. An XL was modified to load from microtiter plates.
In mid 1995, the Corporation conducted another organizational experiment when it was realized that the “Anderson Plan” had damaged the Corporation’s position in all of its markets. The new structure divided the organization into four business segments by product lines: Large Hematology, Small Hematology, Cytometry, and Particle Characterization. Each Business Segment had two co-directors, one responsible for R&D and Strategic Planning and the other responsible for Field Operations. Together, the co-directors were responsible for their business segment. I became the Corporate Director of R&D and Strategic Planning for the Cytometry Business Segment.
In 1996, we began working on a high pressure sorting option for the ELITE. This evolved into a major upgrade of the ELITE platform that was released in 1998 as the ALTRA. In late 1997, it was announced that The Coulter Corporation was being acquired by Beckman Instruments. The deal closed in early 1998. Within a year, most of the senior Coulter executives had left and the business oriented organizational structure was replaced by a functional structure. Most of the cytometry projects that were active at the time of the acquisition were terminated and a lot of talent was lost. I was no longer was involved with the Cytometry Business. In 2001, I was offered a position with the Advanced Technology Center, a corporate level resource charged with satisfying the long term technology requirements of the Corporation. I continue in that capacity today. Four years ago, my wife and I moved to Key Largo where we plan to retire.
Throughout the years, it has been my honor and pleasure to work with many collaborators and customers. If I try to list them all here, I am sure I will embarrass myself by failing to include more than one that was very important. To all of them I just want to say thank you for what you have taught me and what we accomplished together.

- Robert Auer
- auerbob@aol.com

