NK FUNCTION IN FLOW
CYTOMETRY
Marco Vitale
Department of Biomedical Sciences and Biotechnologies
Human Anatomy Section
University of Brescia, via Valsabbina 19, 25123 Brescia
Italy
Tel. +39 30 371-5300
Fax +39 30 370-1157
E-mail: vitale@master.cci.unibs.it
INTRODUCTION
Physical interaction between natural killer
(NK) cells and target cells is a key event in NK cell physiology. Natural
killer cells were defined as cells able to recognize and destroy targets
that have lost expression of MHC class I molecules (1). However, it has
recently been discovered that the molecular machinery that controls NK
cell behaviour towards other cells is more complicate than it was thought
before. NK cell activity appears in fact, finely tuned by the opposing
activity of stimulatory and inhibitory receptors. NK binding to a variety
of target cell triggers its lytic activity through the stimulatory receptors.
This activation can be shut down by the inhibitory receptors, which deliver
a negative signal upon binding to MHC class I molecules on target cells.
NK cells display on their surface receptors specific for MHC-I allotypes
that are able to inhibit target killing (2). It is therefore evident that
the binding to target cells is crucial to drive the subsequent NK activity
towards killing or not. NK cells can kill their targets by different mechanisms:
i) cell-mediated: this can take place through the classical, perforin-mediated
target membrane damage, or through the induction of granzyme-mediated cytotoxicity.
This type of cytotoxicity involves the binding of effectors and targets
as a necessary prerequisite for lysis. ii) via soluble factors: NK cells
can be stimulated to produce IFN-g
and TNF-a
, which can exert cytostatic/cytotoxic effects on specific targets. This
assay does not necessarily involve effector-target cell contact. Flow cytometry
has been successfully employed to measure: 1) binding of NK cells to targets
(3-10); 2) NK cell-mediated cytotoxicity (11-12). It has numerous advantages
upon other techniques which can measure NK binding and cytotoxicity. It
is, in fact, fast, independent from the operator, statistically reliable
and avoids the use of radioactive material. Moreover, the analysis is made
at the single cell level, and not on whole PBL. The two steps of the NK
lytic activity (binding and killing) can be measured separately by flow
cytometry. However, flow cytometry also allows the measurement of binding
and killing at the same time, in a single test tube. The flow cytometry
methodologies that follow describe: i) the measurement of NK binding to
targets; ii) the measurement of NK cytotoxicity by propidium iodide staining
of dead targets; iii) the contemporary measurement of binding and cytotoxicity,
which gives the percentage of target cells effectively killed by the NK
cells bound on their surface.
MATERIALS
1. F(abí)2 fluorescent NK cell
surface marker. In this description we shall use anti-CD56-phycoerythrin
conjugated (PE). The brightest the fluorescence of the mAb, the best the
measurement of binding and cytotoxicity.
2. Ficoll gradient, to separate PBL.
3. Target cells. We shall refer to the K562
human erythroleukemia cell line as the standard target for human NK assays.
K562 must have a viability greater than 95%. (It may be useful to split
them 1:1 the day before use).
4. Propidium iodide
5. Four parameter flow cytometer equipped
with a argon ion laser tuned at 488nm wavelength. Data acquisition in list
mode.
METHODS
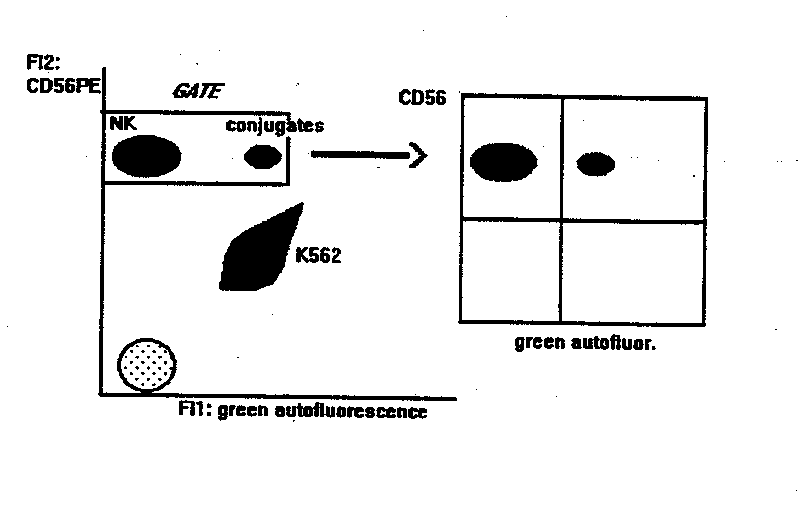
NK BINDING ASSAY
This method gives the percentage of NK cells
bound to targets. Compared to other methods, it is statistically reliable
and allows the double labelling of effector cells, thus identifying subsets
of NK cells (or other effectors) bound to targets.
- PBL separation. Dilute heparinized
blood 1:1 with PBS (or 1:5 if starting from buffy coat). Layer blood on
Ficoll at room temperature. Centrifuge 13 min at 3000 rpm, (no brake).
Collect peripheral blood mononuclear cells, wash them once in PBS and eliminate
monocytes by plastic adherence. Then count PBL.
- mAb staining of effectors. 2x105
PBL are incubated with anti-CD56-PE for 10 min at room temperature. Anti
CD3-Fitc can be added in the tube, to detect the binding activity of T
cells at the same time. If the flow cytometer can detect a third fluorescence
emission > 600 nm (like PerCP or Red 670) or is equipped with a second
HeNe laser to excite APC, PBL can be also stained with mAbs that identify
subsets within the CD56+ NK or the CD3+ populations. At > 600 nm (or under
the excitation of HeNe laser source) in fact, K562 cells are not autoflurescent,
and double stained populations can be identified, as explained below.
When using Fc receptor positive target cells,
F(abí)2 mAb must be used, to avoid Ab-mediated binding.
- wash 3x with cold PBS
- Mix at 1:1 ratio PBL and targets (K562).
Final volume: 300m
l
- Centrifuge at 1000 rpm, 5 min
- place the tubes in a water-bath at 37°C,
5 min
- transfer the tubes on ice, 30 min, gently
resuspending the pellet.
- flow cytometry analysis:
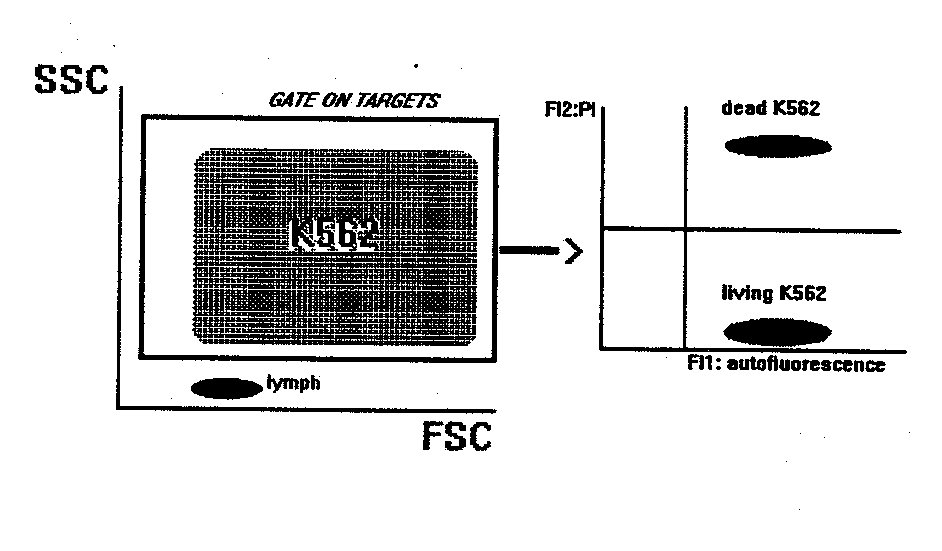
NK CYTOTOXICITY ASSAY
This method gives the percentage of target cells killed by natural cytotoxic
cells present in PBL.
- PBL separation. Dilute heparinized
blood 1:1 with PBS (or 1:5 if starting from buffy coat). Layer blood on
Ficoll at room temperature. Centrifuge 13 min at 3000 rpm, (no brake).
Collect peripheral blood mononuclear cells, wash them once in PBS and eliminate
monocytes by plastic adherence. Then count PBL.
- Mix in a tube target and effector cells at the desired ratios. Final
volume: 300-500 m
l of RPMI1640+10%FCS+1m
g/ml propidium iodide (PI).
-spontaneous lysis: put in a separate tube
only target cells, without effectors. Treat this tube exactly as the others.
-centrifuge tubes at 1000 rpm, 5 min
-incubate tubes for 2 hr at 37°C, 5%
CO2, gently resuspending the pellet. The time of incubation
should however be standardized preliminarly.
-flow cytometry:
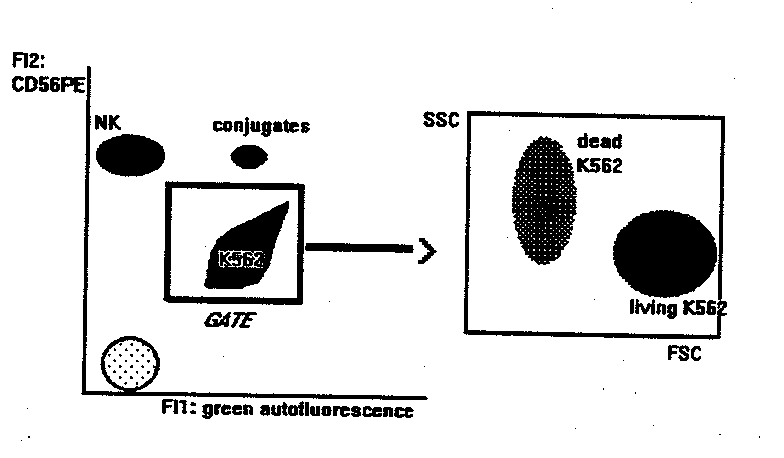
NK BINDING AND KILLING ASSAY
This method measures the binding and killing activities of NK cells
at the same time.
- PBL separation. Dilute heparinized
blood 1:1 with PBS (or 1:5 if starting from buffy coat). Layer blood on
Ficoll at room temperature. Centrifuge 13 min at 3000 rpm, (no brake).
Collect peripheral blood mononuclear cells, wash them once in PBS and eliminate
monocytes by plastic adherence. Then count PBL.
- mAb staining of effectors. 2x105
PBL are incubated with anti-CD56-PE for 10 min at room temperature. Anti
CD3-Fitc can be added in the tube, to detect the binding activity of T
cells at the same time. If the flow cytometer can detect a third fluorescence
emission > 600 nm (like PerCP or Red 670) or is equipped with a second
HeNe laser to excite APC, PBL can be also stained with mAbs that identify
subsets within the CD56+ NK or the CD3+ populations. At > 600 nm (or under
the excitation of HeNe laser source) in fact, K562 cells are not autoflurescent,
and double stained populations can be identified, as explained below.
When using Fc receptor positive target cells,
F(abí)2 mAb must be used, to avoid Ab-mediated binding.
- wash 3x with cold PBS
- Mix at 20:1 ratio PBL and targets (K562).
Final volume: 300m
l of RPMI1640+10%FCS.
- Centrifuge at 1000 rpm, 5 min
- place the tubes in a water-bath at 37°C,
5% CO2 for 2hr. The time of incubation should however be standardized
in preliminary experiments.
- flow cytometry:
REFERENCES
1. Ljunggren HG, Karre K. Immunology Today 11:237-244, 1990
2. Moretta A, Bottino C et al. Ann Rev Immunol 14:619-648,
1996
3. Grimm E, Bonavida B. J. Immunol 123: 2861-2869, 1979.
4. Segal DM, Stephany DA. Cytometry 5: 169-175, 1984.
5. Storkus WJ, Balber AE et al. Cytometry 7: 163-170, 1986.
6. Lebow LT, Stewart CC et al. Nat Immun Cell Growth Regul 5:
221-237, 1986.
7. Garcia-Pennarubia P, Koster FT et al. Nat Immun Cell Growth
Regul 8: 57-65, 1989.
8. Cavarec L, Quillet-Mary A et al. J Immunol Meth 130:251-261,
1990.
9. Vitale M, Zamai L et al. Cytometry 12: 717-722, 1991.
10.Vitale M, Zamai L et al. I Immunol Meth 149: 189-196, 1992.
11.Papa S, Vitale M et al. J Immunol Meth 107: 73-78, 1988.
12.Vitale M, Neri LM et al. J Immunol Meth 121: 115-120, 1989.
Back to advanced methodologies
Back to cell function assays
CD-ROM Vol 3 was produced by Monica M. Shively and other
staff at the Purdue University Cytometry Laboratories and distributed free
of charge as an educational service to the cytometry community. If you
have any comments please direct them to Dr. J. Paul Robinson, Professor
& Director, PUCL, Purdue University, West Lafayette, IN 47907. Phone:(765)
494-0757; FAX (765) 494-0517; Web
http://www.cyto.purdue.edu, EMAIL cdrom3@flowcyt.cyto.purdue.edu