2.1 Instrument sensitivity is verified by:
(1) Using freshly stained lymphocytes, establishing that positivenegative separations are acceptable, OR
(2) If sensitivity particles (e.g. fluorochromelabelled beads or nuclei) are used, run them at testspecific settings established at the time of initial setup.
(3) Record mean fluorescence channel
and CV in the daily log book and/or on LevyJennings
plots (Sensitivity Log).
2.2 Spectral compensation is verified by:
(1) Freshly stained cells using mutually exclusive antibodies, e.g. CD3-FITC and CD19-PE for lymphocytes.
(2) If compensation particles (e.g. FITC- and PElabelled beads) are used, run them at testspecific settings and compensation levels established at time of initial instrument set-up.
(3) 3-colour or 4-colour compensation requires single-colour preparations for each additional fluorochrome.
(4) Record mean channel fluorescence
intensity for each population of interest (red only, green only,
and negative for both) in the daily log book and/or on LevyJennings
plots (Compensation Log).
If particles values are not within acceptable
range, compensation settings should be reevaluated using
antibodystained leucocytes.
Note: Overcompensation leads to
fewer errors than undercompensation.
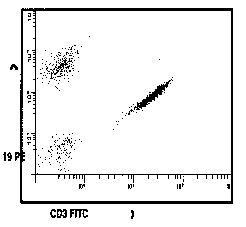
Figure 1
Representation of application of correct
compensation. Gated correlated display of anti CD3-FITC and anti
CD19-PE. (A) Uncompensated. (B) Correctly compensated.
(1) Running a "normal" specimen
stained with an antibody reagent such as anti CD3-FITC and
CD4-PE at testspecific instrument settings.
(2) Verifying acceptable light scatter
resolution of the leucocyte populations.
(3) Verifying that the percentage of
antibodypositive lymphocytes is acceptable by comparison
with previous results, or with established laboratory ranges for
the antigens selected (Appendix 3).
If this positive control does not meet
laboratory criteria, remedial action should be taken. Instrument
performance and/or staining procedure should be checked to determine
the source of the problem. Any problems identified using this
sample must be rectified prior to analysis of test specimens.
1. Sample order. Run and check all control specimens
first, before running the patient samples according to laboratory priority.
2. Test order within any panel. The
first tube should be a gating control to maximise the cells of
interest and minimise contamination. The appropriate isotype controls
should be run next, followed by the subsequent test panel
to investigate the provisional diagnosis.
3. Assessment of specimen viability
is desirable; however, because of biohazard concerns, it is recommended
that all samples be appropriately fixed prior to analysis on the
flow cytometer. It is not presently possible, on a routine large-scale
basis, to distinguish those cells which were non-viable
prior to fixation. However, this can be performed using ethidium
monoazide (EMA) as described by K. Muirhead, 2nd AFCG Methods Course,
1989.
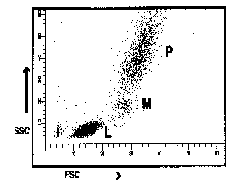
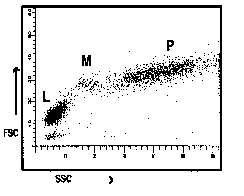
Figure 2
Representation of common ways of displaying
correlated low angle versus 90o angle light scatter
seen from lysed whole blood preparations. (L = predominantly lymphocytes,
M = predominantly monocytes, P = predominantly polymorphonuclear
leucocytes).
4. Set leucocyte gates as broadly as
possible consistent with acceptable levels of contamination to
minimise contaminating cells and maximise the inclusion of the
cells of interest (see Appendix 3).
5. Each laboratory should establish
limits of contaminating cells and debris, based on documentation
that their inclusion does not significantly affect the measurement
of interest. If levels of contamination exceed established laboratory
limits, the corrective actions taken are to adjust the light scatter
gates and reanalyse the immunofluorescent correlated two-colour
plot.
Typical satisfactory values for lymphocytes
are 95% (minimum 90%) of all lymphocytes and 90% (minimum 85%)
purity in the gate as determined by CD45-FITC/CD14-PE gating control
[Appendix 3].
6. If levels of contamination by non-lymphocytes
cannot be minimised to within acceptable limits, then
test results may be suspect.
If this contamination cannot be explained
by reinterpretation of the data or by clinical diagnostic reasons,
a second specimen should be requested.
7. Count at least 2000 gated events
in each sample. This number assures with 95% confidence that
the result is within 2% of the "true" value (binomial
sampling). NB: This sample mode assumes that the variability of
determining replicates is < 2%.
8. The counting of 2000 gated events
to ensure reasonable statistical confidence may not be achievable
in severely leucocytopaenic specimens.
9. Most commercially available directly
conjugated reagents give good resolution between low intensity
negative and higher intensity positive cell populations. When
simultaneous two-colour immunofluorescent correlated data is analysed,
boundaries must be set to define four distinct regions: cells
labelled with neither antibody, cells labelled with antibody #1
but not antibody #2, cells labelled with antibody #2 but not #1,
and cells labelled with both antibodies.
1. The possibility of patients' contesting
the diagnostic implications derived in part from flow cytometric
testing makes it incumbent upon the laboratory to be able to demonstrate
and verify the process used in arriving at the reported test results.
2. Where possible all listmode data
on all samples analysed should be retained.
At a minimum, retain correlated dual
fluorescent data for each test and any interpretive comments on
samples where a significant diagnosis is made.
3. Retain all primary files, worksheets,
and report forms.
4. Minimum duration of data storage
depends on state and federal regulations. These regulations may vary and each
laboratory will need to remain informed of the current requirements.
1. Analysis should include internal
reliability checks of results, including:
a) Optimally, the sum of CD3+% plus
CD19+% plus CD3-CD16+ and/or CD56+ (the "lymphosum")
should equal the purity of lymphocytes in the gate ± 5%,
with a maximum variability of £
10%. If the data are corrected for lymphocyte purity, then the
lymphosum should be between 95 and 105% (minimally 90-110%).
b) Optimally, the sum of the CD3+CD4+%
plus CD3+CD8+% should be no more than 5% more than the CD3+%,
and no more than 10-15% less than the CD3+%, depending on the
number of g/d-TCR+CD3+
cells present.
c) Replicate results within a panel
(e.g. CD3+%) for the same sample should be within 5% of each other
for FS v SS gating or within 3% for CD45 v SS gating.
d) Light scatter patterns should be
examined for each tube within the panel for variation from tube
to tube. Similarly, the number of gated events and/or time to
collect data should not vary greatly from tube to tube.
 (A) .
(A) . 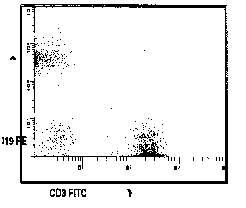 (B)
(B)
3. Overall system performance can be
verified by:
Definition of a lymphocyte gate
Potential sources of error which are
not necessarily covered by the above reliability checks may include
inappropriate gating leading to exclusion of relevant cells, tubes
in a panel run in the wrong order, inappropriate cut-offs between
negative and positive cells, and calculation or transcription errors.
Individual laboratories may require procedures to cover such possibilities.
2. Each laboratory should determine
the level of test variability by preparing and analysing at least
six replicates. This will provide a basis when changes to methodology
are introduced.
Example 1:
A sample control measure is the lymphosum2, which is
the sum of T cell %, B cell %, and NK cell % ; ideally this should
equal 100% for assays corrected for gate purity. Typically, lymphosum
values of 95%105% are acceptable.
Example 2:
Tube-to-tube variation can be monitored by the inclusion of the
same antibody in separate tubes within the one patient test series.
3. Regulatory bodies currently require
that a laboratory keep all equipment maintenance and calibration
records, staff training records, up-to-date method protocols,
daily operator/reagent records, verification of transcription
of results from machine printouts, procedures for amendment of
results, and checks by supervisors/pathologists.
4. Where possible, the laboratory should
belong to and participate in a recognised external Quality Assurance
program with regular review of the results.
1. Report all unique patient identifiers
including name/code, medical record number, laboratory ID/accession
number, and collection date/time as well as print date/time.
2. Report all data in terms of cluster
of differentiation (CD) with a short description of the main antigen
recognition characteristics.
3. For unclustered antibodies, report
the clone name with a short description of the main antigen binding
characteristics.
4. For blood specimens, report all data
as a percentage and absolute number of the population of interest
within the gate as determined by the gating control.
5. Report data from all relevant antibody
phenotyping combinations with corresponding reference limits
of expected normal values, e.g.: CD3+8+ suppressor/cytotoxic
T Cells ± % and/or ± absolute values.
Reference limits for immunophenotyping
test results must be determined for each laboratory.
6. Each laboratory should establish
reference limits for the antigens being tested (see Appendix 1).
1. Universal precautions: There appears
to be no single document that addresses the specific needs of
flow cytometry.
It is recommended that readers refer
to the following documents:
(i) Australian Standard AS 2211-1991, Laser Safety.
(ii) Australian Standard AS 2243.3 1995, Safety in laboratories, Part 3: Microbiology.
(iii) NCCLS M29T, Protection of laboratory workers from infectious disease transmitted by blood, body fluids and tissue.
(iv) MMWR 1988;37(24):37782, 3878.
CDC Update: Universal precautions for the prevention of transmission
of human immunodeficiency virus, hepatitis B virus, and other
bloodborne pathogens in health care settings.
2. NCCLS. Vol 12 No 6.
3. MMWR MARCH 4, 1994 / Vol. 43 / No
RR3. 1994 Revised Guidelines for the Performance of CD4+
TCell Determinations in Persons with Human Immunodeficiency
Virus (HIV) Infection.
4. Muirhead, K.A., Wallace, P.K., Schmitt,
T.C., Rescatore, R.L., Ranco, J.A., Horan, P.K. Methodological
considerations for implementation of lymphocyte subset analysis
in a clinical reference laboratory. In Clinical Cytometry.
M. Andreeff, ed. Ann. N.Y. Acad. Sci. Vol. 468, pp 113127,
The New York Academy of Sciences, New York, N.Y., 1986.
5. Loken, M. R., Meiners, H., Terstappen,
LWM. Comparison of sample preparation techniques for flow cytometric
analysis of immunofluorescence. Cytometry Supplement 2:53, 1988.
6. Schlossman, SF, et al. (eds). Leucocyte
Typing V. White Cell Differentiation Antigens, Oxford University
Press, 1995.
7. Nicholson, JKA, Hubbard, M and Jones,
BM. Use of CD45 fluorescence and side-scatter characteristics
for gating lymphocytes when using the whole blood lysis procedure
and flow cytometry. Cytometry 26: 16-21, 1996.
APPENDIX 1: DETERMINATION OF REFERENCE RANGES
1.0 Definitions
Reference values: Set of values for any measured quantity.
Reference interval: Classically, the
range of values found in 95% of a reference population of healthy
individuals without overt clinical disease.
NOTE: Age, sex, and race are factors
known to influence reference intervals.
2.0 Procedure for Determining Reference
Ranges
Statistical methods, both parametric
and nonparametric, and graphical methods are discussed in detail
in references 13. Only a brief summary of the steps involved
is presented here.
-
2.1 Parametric methods
-
(1) Collect data on randomly chosen
set of representative individuals (e.g. 50 healthy individuals).
(2) Inspect frequency distribution of
values obtained.
(3) If frequency distribution is Gaussian,
use appropriate statistical techniques to estimate 95% confidence
interval and use endpoints of interval as the reference range.
(4) If frequency distribution is nongaussain,
back transform endpoints of 95% confidence interval to obtain
reference range, (e.g. log X, of (X + C), square root X, arcsin
X), and proceed as in step 3.
(5) If no satisfactory transformation
can be identified, use nonparametric methods which do not depend
on the exact distribution of the data.
2.2 Nonparametric methods
-
(1) Collect data on randomly chosen
set of representative individuals.
(2) Arrange data in ascending
or descending order.
(3) Use appropriate nonparametric
techniques to identify desired limiting percentiles (e.g. 2.5
and 97.5) to desired confidence level.
Nonparametric methods are most appropriate
when data do not show a Gaussian distribution and cannot be
so transformed. However, they are very sensitive to outliers,
and final ranges chosen may be highly dependent on methods used
for removing outliers (13).
3.0 Pitfalls in Determining Flow
Cytometric Reference Ranges
Each laboratory should determine its
own reference range using its particular preparation method and
instrumentation, because significant laboratorytolaboratory
differences related to these variables have been reported.
However, quite large data sets are technically
required to carry the above described methods for reference range
determination, typically >300 for parametric methods and >120
for establishing nonparametric interval with 90% confidence. Until
more standardised methodology allows pooling of data among laboratories
(hence this document), this is clearly an unrealistic expectation.
Other confounding variables besides
sample size have been described (45).
One practical solution to the dilemma
is to accumulate and analyse reference data in smaller sets (e.g.
1020 individuals), which can then also be pooled and analysed.
If the last two sets of pooled data are found to give the same
reference range within experimental error, this gives increased
confidence that the reference range selected is not unduly affected
by the small sample size.
REFERENCES FOR APPENDIX 1
1. Winkel, P., Statlan, B.E. Reference
values. In Clinical Diagnosis and Management by Laboratory
Methods (ed J.B. Henry), Philadelphia, W.B. Saunders Co., 1979,
pp. 2952.
2. Martin, H.F. Gudzinowicz, B.J. Fanger,
H. Normal Values in Clinical Chemistry, New York, Marcel
Dekker, 1975, pp. 102236.
3. Henry, R.J., Cannon, D.C., Winkelman,
J.W. Clinical Chemistry. Principles and Technics, New York,
Harper and Row, 1974, pp. 343371.
4. Edward, B.S., Altobelli, K.K., Nolla,
H.A., et al. A comprehensive quality assessment approach for flow
cytometric immunophenotyping of human lymphocytes. Cytometry
10:443441, 1989.
5. McCarthy, R.C., Fetterhoff, T.J.
Issues in Quality Assurance in Clinical Flow cytometry. Arch. Pathol.
Lab. Med.113: 658666, 1989 (in press).
Back to standards and regulations
Back to consensus documents and ring trials
 |
 |
 |
 |
 |
CD-ROM Vol 3 was produced by Monica M. Shively and other staff at the Purdue University Cytometry Laboratories and distributed free of charge as an educational service to the cytometry community. If you have any comments please direct them to Dr. J. Paul Robinson, Professor & Director, PUCL, Purdue University, West Lafayette, IN 47907. Phone:(765) 494-0757; FAX (765) 494-0517; Web http://www.cyto.purdue.edu, EMAIL cdrom3@flowcyt.cyto.purdue.edu