CD4/CD8 ABSOLUTE COUNTS NOW
AVAILABLE ON COULTER HEMATOLOGY SYSTEMS

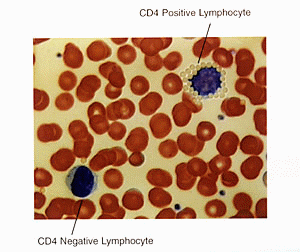
MIAMI, FL - Marking the first time that lymphocyte subset analysis is available on a conventional hematology analyzer, Coulter Corporation announces the availability of CD4 and CD8 positive lymphocyte enumeration on the COULTER®STKS Hematology System. The company plans to offer this capability in the future on the new COULTER® GEN•S™ Hematology System as well
The innovative monoclonal bead technology is called the CD4/CD8 Cyto-Spheresä Bead Technology Method. In a simple, five-minute procedure, latex beads coated with a specific monoclonal antibody are mixed with the patient blood sample using a unique closed-vial transfer device, completely eliminating operator exposure to blood. When antibody-coated beads come into contact with a cell that has the targeted antigen, the two bind and form a bead-coated rosette. The positive lymphocytes with beads attached are readily distinguishable from other lymphocytes using Coulter’s patented VCS (Volume, Conductivity, Light Scatter) cell differentiation technology.
The ability to easily and safely perform CD4/CD8 testing on an automated hematology system is a breakthrough that offers laboratories the capability to increase testing capacity and reduce turn-around time. Laboratories can move routine and STAT CD4/CD8 testing onto their hematology analyzer, thereby improving instrument utilization and increasing test revenues.
Laboratories can also improve the quality of their CD4/CD8 test results. By removing the additive effect of multiple CV%s from the WBC, LY% and CD4%, the new bead technology provides lower run-to-run CVs than existing methodologies.
A few years ago, Coulter added reticulocyte analysis capability to its VCS-based hematology systems. Now, with the added capability to perform CD4/CD8 assays, Coulter Corporation once again demonstrates that the unique VCS technology employed by its hematology systems is open ended and expandable. Using similar monoclonal bead technology applications, it is possible that the capability to perform other leukocyte subset assays may be added to Coulter hematology systems in the future.
This newest breakthrough strengthens Coulter Corporation's position as a leading provider of automated laboratory systems. The privately held 35-year old company based in Miami, Florida, is the world leader in blood cell analysis.








