Results
Sample A
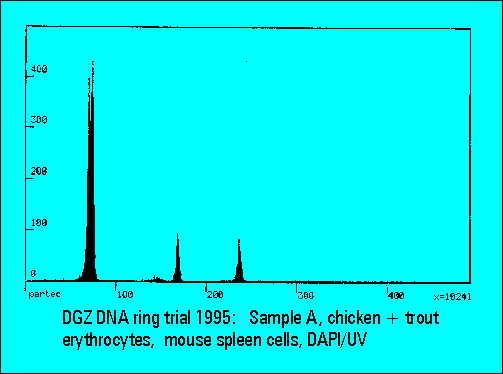
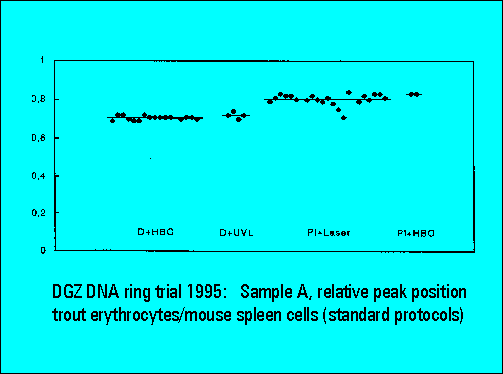
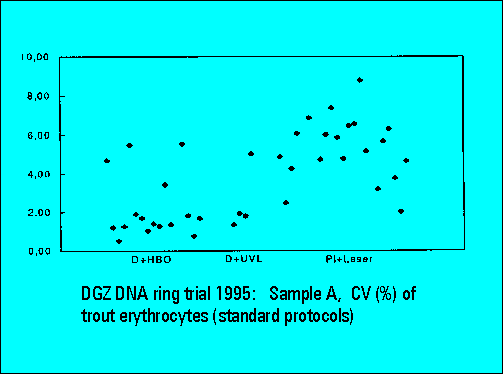
Ring Trial 1995
Organization and sample preparation:
Dr.F.J.Otto
Fachklinik Hornheide
Dorbaumstr.300
D-48174 Münster
Tel: +49/251/3287-651
Fax: +49/251/3287-299
Participating laboratories: 43
Provided samples: 4
- A: chicken and trout erythrocytes with normal mouse spleen cells
- B: trout erythrocytes with DNA-diploid human HSB-2 cells
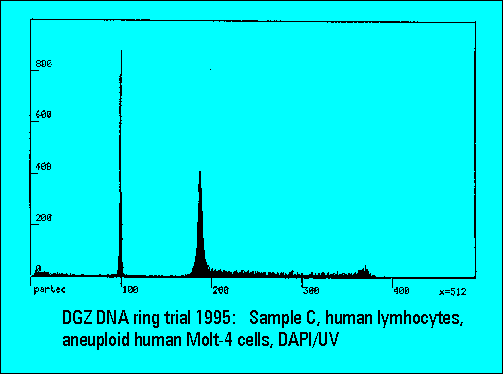
- C: human lymphocytes with DNA-aneuploid MOLT-4 cells
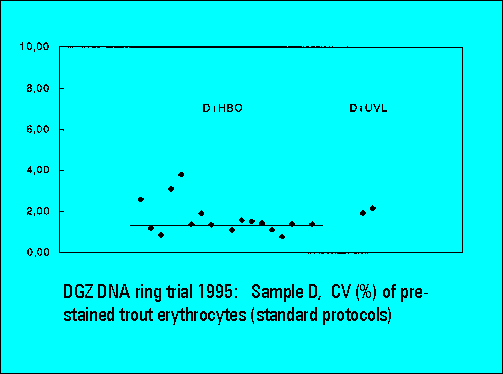
- D: DAPI prestained trout erythrocytes
- all cell samples were fixed in suspension with 80% ethanol
Standard Protocol PI (proposal Th.Liedl)
- centrifuge sample
- remove supernatant by suction
- resuspend pellet in staining solution by stirring with plastic tip pipette (no vortex)
- incubate at 37C for 30min
- measure after 5 min
- if necessary, stained samples may be preserved over night in a refrigerator
- PI staining solution was provided, keep at -20 to -80C for storage (50ug/ml)
PI (Sigma P 4170) with 1mg/ml RNAse (type I-A, Sigma R 4875)
Standard Protocol DAPI (proposal F.J.Otto)
- centrifuge 1-2ml cells for 10min at 200xg
- remove supernatant by suction
- resuspend pellet in 1ml pepsin solution with plastic tip pipette (no vortex)
- incubate at room temperature for 15min whith gentle shaking
- assay 0.5ml cell suspension with 4.5ml DAPI solution
- incubate over night at room temperature
- dissolve one pepsin tube (50mg, Riedel-de-Haen Nr.20821) with 10ml
0.1N HCl prior to use
- dissolve 5.9g tri-NaCitrate2H2O and 0.20mg DAPI in 100ml distilled water.
The solution is stable over several weeks when stored at 4C in the dark.
User Defined Protocols:
- the participating laboratories were free to stain and measure
samples A-D by their own staining protocols in addition to
sample processing by the standard protocols. While the user defined
protocols provided in principle similar results as the standard protocol,
the most consistent results were obtained by the standard protocol procedures.
For reasons of shortness, only the standard protocol results are
displayed in the subsequent panels.
Laboratories Submitting Results:
- PI + argon laser (PI+laser): 23
- PI + HBO lamp (PI+HBO): 2
- EB+M + argon laser: 2
- YOYO + argon laser: 1
- DAPI + UV-laser (D+UVL): 4
- DAPI + HBO lamp (D+HBO): 18
- Hoechst 33342 + HBO lamp: 1



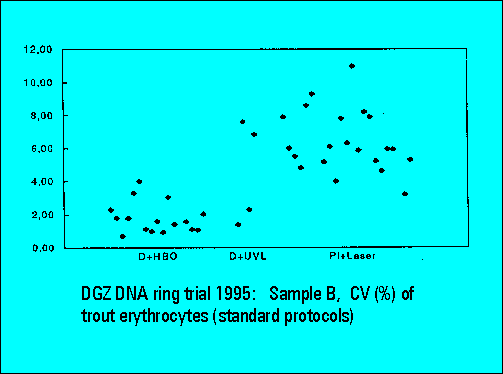
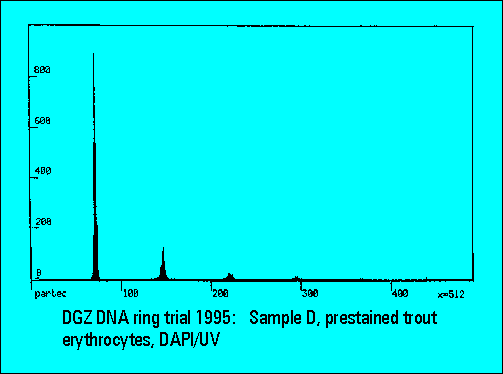
- The instruments should resolve the two narrowly spaced peaks on the left side of the histogram while the two rightmost peaks should not substantially differ. Only few laboratories were able to resolve the two peaks. The CV's (coefficients of variation (%)) were lower for DAPI stained samples measured either by the HBO-100 lamp or by a UV-laser.
- The relative peak position of trout erythrocytes was lower for DAPI than for PI stained samples, confirming earliert ring trial experience. This is explained by the known low AT-content of trout erythrocytes. The ratio calculation between the position of trout and mouse spleen cells was used for interlaboratory data normalization and shows a very low scatter of the values between individual laboratories.
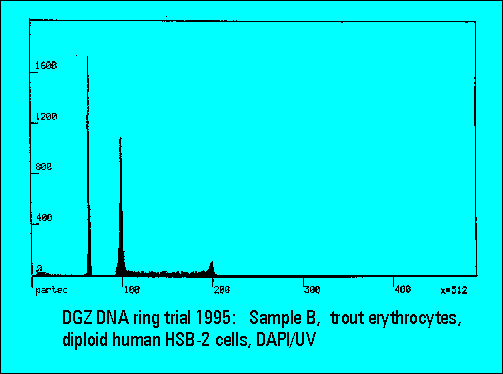
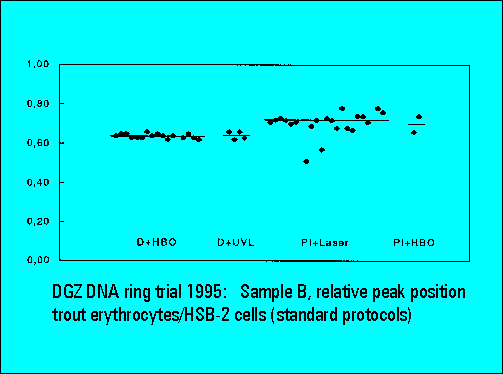
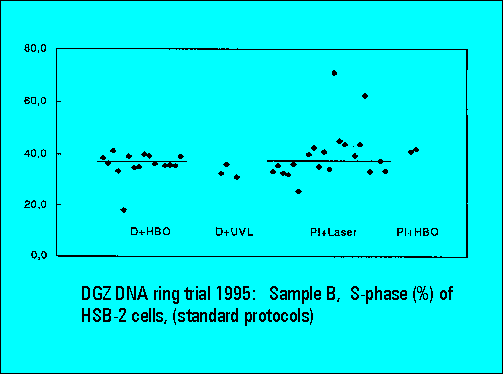
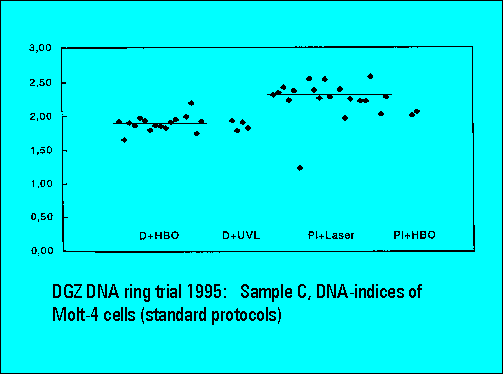
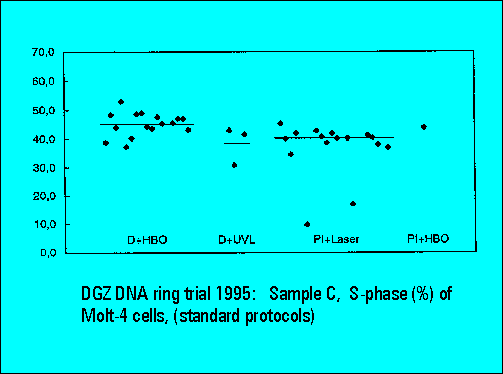
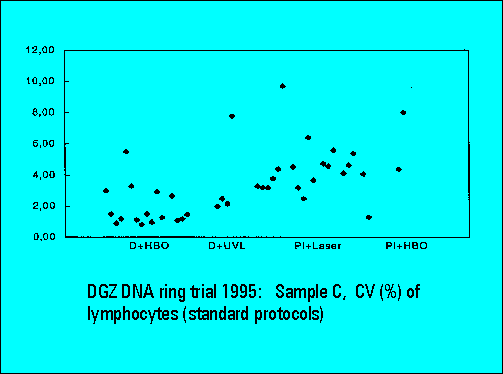
- S-phase values of MOLT4-cell scatter significantly. This is similar to the results of Sample B.
 |
 |
 |
 |