Detection, productivity and growth of the smallest free-living eukaryote using flow cytometry
by Eric Fouilland1, Claude
Courties2, Marie-Josèphe Chrétiennot-Dinet2, Chantal Descolas-Gros3,
Yves Collos4
and André Vaquer4
1 The Scottish Association for Marine Science, Dunstaffnage Marine
Laboratory, UK, erfo@sams.ac.uk
2 Laboratoire Arago, UMR 7628 & 7621 CNRS, Banyuls-sur-mer, France
3 ISEM Paléoenvironnements, UMR 5554 CNRS, Montpellier, France
4 Ecosystèmes Lagunaires, UMR 5119 CNRS, Montpellier, France
Corresponding author: Eric.Fouilland@sams.ac.uk
The smallest known eukaryote, Ostreococcus tauri Courties et Chrétiennot-Dinet [1,2] (Figure 1), measuring <1 µm in diameter, has been discovered in a marine coastal Mediterranean lagoon (Thau Lagoon, France), where it numerically dominates the phytoplankton community throughout the year [3].
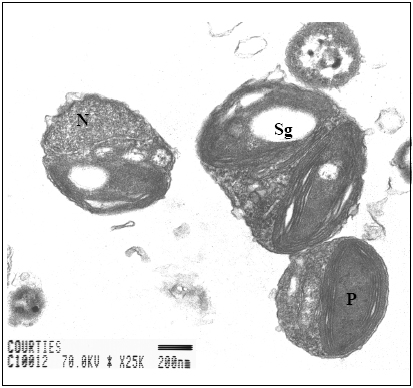
FIGURE 1. Transverse section of single Ostreococcus tauri cells from transmission electron microscopy (N: nucleus, P: Chloroplast, Sg: Starch grain).
Similar forms are beginning to be reported in other coastal areas [4,5]. Because of its small size, this microalga, barely visible by epifluorescence microscopy, has been initially detected by flow cytometry [1] as shown in Figure 2, using low forward angle light scatter (related to cell size) and low red fluorescence from its chlorophyll a content (660-700 nm).
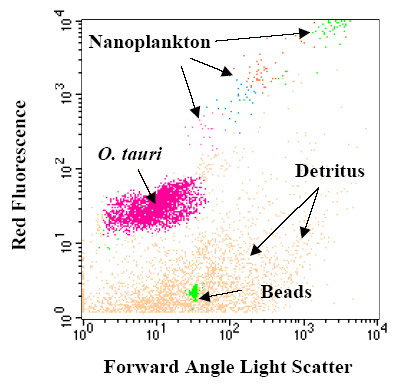
FIGURE 2. Flow cytometric measurements of phytoplankton assemblage in Thau lagoon. Phytoplanktonic cells were distinguished according to their forward angle light scatter (xaxis) and red fluorescence (y-axis) using FACScan flow cytometer (Becton Dickinson, San Jose, CA). One µm beads are systematically used as internal standard.
Very little is known about the growthand ecology of this tiny microalga.
ation of flow cytometric detection and radioactive carbon uptake measurements
was performed on sizefractionated (1 µm) natural planktonic community
submitted to N-nutrient deficiency and sufficiency during a 24-h in
situ incubation in Thau Lagoon. Very high specific productivity (920-1120
fgC cell-1 d-1) and growth rates (3-8 d-1)
of Ostreococcus tauri have been estimated after Nnutrient
enrichments during this study [6]. Such growth rates are consistent with
previous observations for O. tauri in culture and are the highest
ones reported for phytoplankton in the field. This may be related to the
unusual amounts of photoprotective pigments (violaxanthin) measured in
this microalga [1], suggesting a strong adaptation to the high irradiance
levels reached in these shallow waters.
Variations in apparent cell size (forward angle light scatter) of O. tauri were also followed during this 24-h experiment and showed a possible arrest in the cell division of this microalga (no diel size minimum) occurring under N-nutrient deficiency (Figure 3).
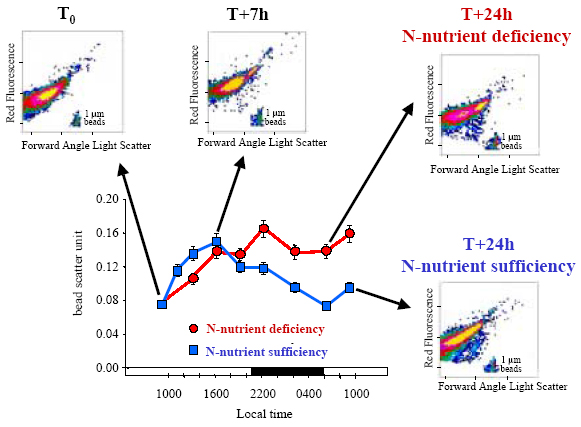
FIGURE 3. Variations in apparent cell size (forward angle light scatter) normalised with 1-µm beads (bead scatter unit) and cytograms of streococcus tauri after 1-µm filtration of natural planktonic community from Thau Lagoon and submitted to N-nutrient deficiency and sufficiency (i.e. nitrate and ammonium enrichments) during 24-h in situ incubations [6]. Flow cytometric analyses were performed using an arc lamp ACR 1000 flow cytometer (Bruker Spectrospin, Wissembourg, France).
The development of an other species with slightly larger cell size under N-nutrient deficiency is unlikely as no eukaryotic species with cell size strictly <1 µm has been recorded other than O. tauri in Thau Lagoon since its discovery, 10 years ago. The short-term metabolic response of O. tauri to the fluctuating nutrient conditions, leading to a very high growth rate under nutrient sufficiency, explains the numerically dominance of this species in the Mediterranean Thau Lagoon, characterised by high inorganic Nnutrient fluxes from shellfish farming.
Acknowledgements
The figure3 was adapted from Fouilland et al. (2004) with kind permission
of Springer-Verlag.
Cited references
[1] Courties et al. 1994. Nature 370: 255.
[2] Chrétiennot-Dinet et al. 1995. Phycologia 34: 285-292.
[3] Vaquer et al. 1996. Limnol. Oceanogr., 41: 1821-1828
[4] O’Kelly et al. 2003. J Phycol, 39:850-854
[5] Worden et al. 2004. Limnol. Oceanogr. 49: 168-179.
[6] Fouilland et al. 2004. Microb. Ecol. DOI: 10.1007/s00248-003-2035-2