Fluorescence Quantitation by Flow Cytometry
 |
 |
|||||||
|
||||||||
 |
 |
View this document as a Microsoft Word file
Definition and introduction
Fluorescence quantification by Flow Cytometry is defined as the measurement
of the intensity of staining of cells and provides an absolute value for
the light intensity it measures.
Quantification of fluorescence is performed comparing cell fluorescence
with a known external standard. By using different beads commercially
available, it is now possible to measure the quantity of fluorescence
relative to the peak channel obtained by flow cytometry, using a standard
curve for its calculation.
There are two units to express the fluorescence quantification, the ABC
(Antigen Biding Capacity) and the MESF (Molecules Equivalent Soluble Fluorochrome).
Applications of quantitation
The quantitation of fluorescence molecules by flow cytometry provides
additional information which is useful for the precise characterization
of cells.
It is useful in hematology for the identification of hemopoietic cell
populations, both normal and leukemic. In certain hematological malignancies,
precursor cells associated antigens can be under or over expressed on
the malignant cells and can therefore be regarded as a leukemia-associated
feature when compared with the normal counterparts. [6]
There is also a hierarchy of expression that is characteristic of different
lineage, i.e.: dim expression of CD4 in monocytes and brighter expression
in lymphocytes.
Some antigens show unimodal expression on blood cell populations, other
antigens may be heterogeneously distributed with different densities.
This may indicate functionally different subsets in relation to a particular
differentiation stage of the cells.
The values of antigen density, when added to percentages or absolute counts
of positive cells, exploit the informative value of the immunofluorescence
test in the interest of defining both normal and abnormal differentiation
pathways and subset compositions as well as signs of cellular activation.
[4]
Quantitative flow cytometry has been used in the study of maturation processes
looking at differentiation antigens, in activation of neutrophils and
complement receptors, in functional assays of adhesion molecules, in infections
looking at the expression of virus receptors, in oncogene products, drug
receptors, steroids receptors, immunophenotyping and pathological situations.
Procedure
Type of quantification beads' kit
Preparation of specimen and beads
Data acquisition
Calculation of ABC or MESF values
Type of quantification beads' kit
The choice of the type of beads depends on the procedure used for the
sample preparation.
The general principle of fluorescence quantification by beads is the same
for different methods of staining of cells.
This is based in commercially available kits. In these kits, there are
usually two tubes. One tube with a mixture of four beads, with four different
levels of uptake of fluorescence, one very dim, one very bright and two
intermediates, and another tube with a blank, i.e. beads with no uptake
of fluorescence.
Direct immunofluorescence
QSC (Quantum Simply Cellular): These are beads coated with goat
anti mouse immunoglobulins, each level of the standard can bind to a certain
amount of mouse Ig. They are used to quantify direct immunofluorescence.
They measure ABC (Antibody Binding Capacity), when saturated with the
same fluorochrome conjugated monoclonal antibody as used on the cell sample.
These beads require one calibration for each monoclonal antibody.
Quantibrite: These are beads manufactured only by Becton & Dickinson to be used with their Cell Quest program Quanti Quest. These are beads labeled only with PE. There are not many McAb available at the moment. BD claims that they can label the PE at a ratio 1 to 1.
Indirect Immunofluorescence
QIFIKIT these are beads coated with monoclonal antibodies which
mimic monoclonal antibody bearing cells. These receptors bind to the secondary
antibody used both to stain the cells and the beads, therefore they require
only one calibration per experiment.
Direct and Indirect immunofluorescence
FCSC (Quantum beads): These beads are coated with known molecules
of fluorochrome, they are available conjugated to FITC or PE and measure
the MESF. The fluorescence of the cells is compared to standard molecules
of fluorochrome.
Preparation of specimen and beads
The samples should be prepared as usual by the method of choice of the
laboratory.
Please note that if one is doing intracytoplasmic staining, the beads
should not undergo the process of fixation and permeabilisation.
i. Direct immunofluorescence:
The beads are incubated with the relevant McAb to be quantified for one
hour at 4oC. Kits used for this methodology are: Quantum Simply Cellular,
Quantibrite and FCSC Quantum Beads.
The specimen is prepared following the standard in house protocols.
One standard curve is required for each McAb to be tested.
ii. Indirect immunofluorescence:
The beads are stained at the stage of applying the secondary antibody,
they are incubated for one hour at 4oC. The cells of the specimen, are
stained following the in house protocols.
One standard curve is sufficient for different McAb tested, provided that
the same secondary antibody was used.
The monoclonal antibody is not directly conjugated to a fluorochrome and
a second incubation with a fluorescent antibody against the primary is
needed to detect the reaction by flow cytometry. The secondary antibody
can be labeled with one fluorochrome, i.e.: FITC, PE or Third color. [1]
Fig. 1
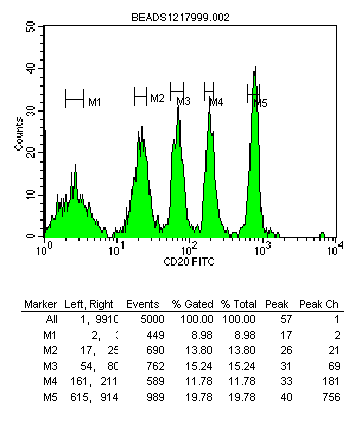
Fig. 1: Histogram showing blank and 4 fluorescence peaks. Statistics showing
peak channel values
Quantification rules:
There are three essential rules to perform quantification:
a. The monoclonal antibody has to be applied at saturating amounts both
for the beads and the cells in the specimen.
b. The same reagent , from the same company and at the same dilution should
be used throughout the experiment and other future analysis.
c. The instrument fluorescence setting should be maintained unchanged
once the beads have been run and the analysis of the unknown sample should
be acquired with the same setting.
Data acquisition
Acquiring beads:
The tube with the beads is acquired firstly in all cases where one experiment
is sufficient for the quantification, this is the case for the Quantum
beads and the QUIFIKIT beads. In the case of the Quantum Simply Cellular,
where a tube is run for each monoclonal antibody, the tube with the beads
for that particular McAb should be run first, and then all the other tubes
with beads for the other different McAb.
The SSC voltage needs to be decreased more than that for cells in order
to bring the beads into the FSC/SSC dot plot. There could be some doublets
if the tube was not shaken vigorously, these are excluded by doing a tight
gate around the beads, data is acquired in these gated beads.
The instrument should be set up in a way that the fluorescence signal
of the tube with the blank (unlabelled) beads is located in the region
between 0 and 101, and four other peaks of fluorescence should be seen
along the axis of the relevant fluorochrome. Once the fluorescence voltage
of the instrument is set up, these settings are maintained throughout
the rest of the analysis of the unknown samples. In the case of the QSC,
the settings for each McAb should be used accordingly.
Acquiring the sample:
The SSC voltage will need to be icreased to obtain the cells profile within
the FSC/SSC dot plot.
The settings for the fluorescence channels used for the beads should be
maintained throughout the rest of the analysis of the tubes of the sample.
Calculation of ABC or MESF values
Relevant software is provided with the quantification kits. These programs
are user friendly. The data obtained from the cytometer is put into the
program and automatically a standard curve is produced. These programs
take into consideration the make of the instrument used, the voltage for
that sample, the fluorochrome used, the supplier of the McAb, etc.
The program calculates the ABC and/or MESF value of the unknown sample
and saves the data.
The standard calibration curve is obtained by plotting the values of the
peak channels of the blank and the other four peaks obtained from the
flow cytometer against the assigned known number of molecules of fluorochrome
obtained from the supplier of the beads.
The peak value of the unknown sample is obtained by running the sample
with the same fluorescence setting as the beads minus the peak value of
the control tube (tube with the isotypic conjugate fluorochrome but with
no primary antibody)
Using the software provided with the beads, fill in the relevant data
and enter the peak channels of the different peaks for the beads obtained
from their analysis on a histogram plot to obtain a standard curve.
Pitfalls of the method:
One should have careful consideration to the fact that the volume of McAb
needed to saturate the cells sample, is not necessarily the same as required
to saturate the beads and a greater volume of McAb may be needed to saturate
the latter.
The position of the fluorescence setting of the blank may have to be negotiated
and set up slightly lower or higher than the ideal situation just within
the 101 limit. Some samples have very dim fluorescence and if the blank
is set up too low, the intensity of the sample may be difficult to calibrate.
Overestimation of cell bound ABC values can occur when QSC beads are used
because epitopes on cells are homogenous and of high affinity while bead
bound GAM Ig bind mouse Ig with variable affinity.
Quantification clinical applications in hematology:
Acute Lymphocytic Leukemia:
The leukemic cells represent the malignant counterparts of normal hematopoietic
precursors expressing terminal deoxynucleotidyl transferase (TdT), CD10
and CD19.
Normal TdT+ precursors have significantly higher number of TdT (>100x
103) and lower number of CD10(<50x103) and CD19 (<10x103).than in
B lineage ALL blasts TdT (<100x103) CD10 >50x103) and CD19 (>10x103)
molecules per cell. These differences were statistically highly significant,
therefore, the quantitative analysis of TdT combined with CD10 and CD19
may allow a clear distinction between normal precursors and minimal residual
leukemia in B-lineage ALL and avoid the pitfall of misinterpreting regenerating
B-cells as evidence of relapse.[2]
Quantification of B-cell antigens:
The B-lymphocytes of B cell chronic disorders have their counter part
in the normal B-cells of the peripheral blood. Several published works
studied a series of B-cell antigens in to assess if there was any difference
between the normal B-cell in the blood and those B-cells from different
chronic B-cell disorders. Also to assess if there was any distinct expression
pattern in antigen binding capacity amongst different B-cell disorders
to help in diagnosis, detection of minimal residual disease, and sometimes
treatment. [7]
Quantification of T-cell antigens:
Most T-cell antigens are expressed on normal and neoplastic T lymphocytes
and for this reason it is not easy to distinguish between the immunophenotype
of normal and malignant T-cells.
There are results published that show that the quantitative analysis of
CD3 and CD7 and their combined evaluation may enable a distinction between
normal and leukemic T-cells and could facilitate the monitoring of minimal
residual disease.
The study has also defined the T prolymphocyte as a cell of intermediate
maturity between thymus derived and peripheral T lymphocytes.[3]
Conclusions:
Quantification is a useful methodology to extract additional information
from the flow cytometer.
This extra information is helpful in hematological malignancy and provides
a refined knowledge of hemopoietic differentiation, permits a more objective
definition of positivity and finally it helps to ascertain malignancy,
and normality.
It has also been shown that it provides an additional parameter for the
detection of MRD, particularly in B-cell ALL where the outcome of treatment
could be predicted.[5]
References
1. Poncelet, P., George, F., Papa, S., and Lanza, F.
Quantitation of hemopoietic cell antigens in flow cytometry.
European Journal of Histochemistry. 40/Suppl. 1. 15-32 (1996)
2. Farahat, N., Lens, D., Zomas, A., Morilla, R., Matutes, E., and Catovsky,
D.
Quantitative flow cytometry can distinguish between normal and leukemic
B-cell precursors.
British Journal of Hematology 91, 640-646 (1995)
3. Ginaldi, L., Matutes, E., Farahat, N., De Martinis, M., Morilla, R.,
and Catovsky, D.
Differential expression of CD3 and CD7 in T-cell malignancies: a quantitative
study by flow cytometry. British Journal of Hematology 93, 921-927 (1995)
4. Bikoue, A., George, F., Poncelet, P., Mutin, M., Jannosy, G., and
Sampol, J.
Quantitative analysis of leukocyte membrane antigen expression: Normal
Adult values.
Cytometry ( communications in Clinical Cytometry) 26: 137-147 (1996)
5. Farahat, N., Morilla, A., Owusu-Ankomah, K., Morilla, R., Ross Pinkerton,
C., Treleaven, J., Matutes, E., Powles, R., and Catovsky, D.
Detection of minimal residual disease in B-lineage acute lymphoblastic
leukemia
by quantitative Flow Cytometry.
British Journal of Hematology 101: 158 164 (1998)
6. Ginaldi, L., De Martinis, M., Matutes, E., Farahat, N., Morilla, R.,
and Catovsky, D.
Levels of expression of CD19 and CD20 in Chronic B-Cell leukemias.
Journal of Clinical Pathology. Vol 51: 364-369 (1998)
7. D'Arena, G., Musto, P., Cascavilla, N., Dell'Olio, M., Di Renzo, N.,
and Carotenuto, M.
Quantitative flow cytometry for the differential diagnosis of leukemic
B-Cell chronic lymphoproliferative disorders.
American Journal of Hematology 64:275-281 (2000)