|
|
Introduction:
The CMV pp65 antigenemia assay is a valuable tool in the diagnosis and monitoring of active CMV
infection in solid organ and bone marrow transplant patients as well as in the diagnosis and
monitoring of CMV disease in AIDS patients. Early and rapid diagnosis of CMV infection is of great
importance in avoiding over treatment with immunosuppressive drugs and in guiding antiviral therapy.
The CMV PO™ antigenemia Kit uses the well defined C10/C11 antibody cocktail to detect the CMV lower
matrix phosphoprotein (pp65), an early antigen in virus replication, which is abundantly present in
antigen-positive polymorphonuclear cells. The CMV PO™ Kit, is a new version of the first FDA
registered immunofluorescence antigenemia kit for in vitro CMV diagnosis, the CMV Brite™ Kit (1).
The development of the CMV PO™ Kit makes quantitative determination of active CMV infection possible
and has been developed for those laboratories who prefer to detect pp65 positive leukocytes by light
microscopy.
Special features of the CMV PO™ antigenemia Kit
- Quantitative assay
- Reading with ordinary light microscope.
- A complete kit containing all the reagents needed from cell isolation to peroxidase staining.
- A cocktail of antibodies specific for pp65. Antibodies C10/C11 are highly specific for pp65 and have been well documented (2,3,4,5).
- Detection of CMV pp65 positive PMNs by the indirect immunoperoxidase assay gives high sensitivity and easy reading of the results.
- The number of CMV pp65 positive cells are counted per duplicate stain.
- Shelf life of at least 18 months.
- Completed within 5 hours of sample collection
- Store cytospots at room temperature. Results can be read several months later
Materials and reagents supplied in the CMV PO™ Kit.
- Dextran Solution
- Erythrocyte Lysing Solution
- Fixative solution
- Permeabilization solution
- Mouse monoclonal antibody C10/C11 specific for CMV pp65
- PO conjugated rabbit anti-mouse immunoglobulin
- AEC substrate solution and acetate buffer
- Hydrogen peroxide solution and Mayer's Haematoxylin solution
Sensitivity and Specificity
The CMV PO™ Kit shows the same high sensitivity and specificity as has been found for the CMV Brite™
Kit (1). There is no evidence of cross reactivity between other viruses and CMV using the CMV
antigenemia assay using the C10/C11 monoclonal antibody coctail.
Results using CMV PO™ Kit
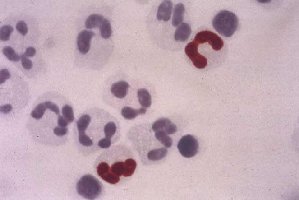 Figure 1 Leukocytes stained with the CMV PO™ antigenemia Kit. Cells containing CMV pp65 antigen
stain dark red-brown and negative cells
are counterstained blue.
Figure 1 Leukocytes stained with the CMV PO™ antigenemia Kit. Cells containing CMV pp65 antigen
stain dark red-brown and negative cells
are counterstained blue.
References
1. Landry,M.L. et al. Evaluation of CMV Brite Kit for detection of Cytomegalovirus pp65 antigenemia in peripheral blood
leucocytes by immunofluroescence. J.Clin.Microbiol. 34. 1337-1339
2. Van Son, W.J., The,T.H., (1989) Cytomegaovirus infection after organ transplantation: an update with special emphasis on
renal transplantation. Transpl. Int. 2, 147-164.
3. The, T.H., et al, (1990) Cytomegalovirus antigenemia. Rev. Inf. Dis. 12., S737-S744.
4. Van der Bij et al., (1988) Comparison between viremia and antigenemia for detection of cytomegalovirus in blood. J. Clin.
Microbiology 26. 2531-2535
5. Grefte, J.M.M et al (1992) The lower matrix protein pp65 is the principal viral antigen present in peripheral blood leukocytes
during an active cytomegalovirus infection. J.Gen. Virol. 73. 2923-2932
For research use only. Not for use in humans. For in vitro use only.
|





 Figure 1 Leukocytes stained with the CMV PO™ antigenemia Kit. Cells containing CMV pp65 antigen
stain dark red-brown and negative cells
are counterstained blue.
Figure 1 Leukocytes stained with the CMV PO™ antigenemia Kit. Cells containing CMV pp65 antigen
stain dark red-brown and negative cells
are counterstained blue.
 IQ Products
IQ Products