 |
 |
 |
 |
MoFlo achieves its superior sorting performance by a combination of the following design features:
Without accurate detection and high signal to noise ratio, even at high flow velocity and high event rates, accurate sorting is limited to bright, easily resolved particles. For accurate detection even of dimmer particles, stable, precise alignment and efficient collection optics are the key design issues. For detection of fast-moving, frequent events, fast signal processing and the ability to accept signals from multiple beams is key, and for isolation of very rare particles extremely low correlation errors are essential. Hardware lookup tables are essential to allow sort decisions to closely match the distributions of the target populations, rather than approximating their shapes by simple high/low limits. Particles coincident with the cells of interest must be able to be rejected or included as required, accurately and consistently; fifteen modes of coincidence-handling are provided. Finally, sorting at high speed is not possible without precise, stable control of droplet formation, charging and deflection; and physical separation of closely-spaced events requires high frequency droplet formation, which in turn requires high pressure fluidics and attention to flow cell design for stability and cell viability.
Only all of the above factors in combination can deliver the combination of speed, purity, viability and yield for which MoFlo was designed, and only MoFlo was designed with all these factors in mind.
What cannot be well resolved by the optical detection system cannot be reliably sorted. It is therefore vital that alignment be as easy and as stable as possible. Alignment of MoFlo is unique in that each laser beam is independently controlled at the illumination point by a three axis positioning stage. The flow cell and nozzle are mounted on a five axis positioning stage and the forward scatter detector is equipped with its own three axis stage. On each axis of each stage is a precision (1 micron or .1 micron) Newport-Klinger micrometer. The sequence of alignment is simple and logical:
 using only its independent stage, bring the vertical fluid stream to the (fixed) focus of the collection objective (for safety, a dedicated infra-red camera displays the stream, the flow nozzle and the collection path pinholes on a video monitor);
using only its independent stage, bring the vertical fluid stream to the (fixed) focus of the collection objective (for safety, a dedicated infra-red camera displays the stream, the flow nozzle and the collection path pinholes on a video monitor);
 using only its independent stage, bring laser beam 1 to the stream;
using only its independent stage, bring laser beam 1 to the stream;
 iteratively optimize stream location and beam 1 intersection by fluorescence pulse and/or dotplot monitor with alignment particles;
iteratively optimize stream location and beam 1 intersection by fluorescence pulse and/or dotplot monitor with alignment particles;
 optimize the forward scatter detector position with its independent stage;
optimize the forward scatter detector position with its independent stage;
 using only its independent stage, bring beam 2 to the stream and optimize;
using only its independent stage, bring beam 2 to the stream and optimize;
 using only its stage, bring beam 3 to the stream and optimize.
using only its stage, bring beam 3 to the stream and optimize.
This simple sequence and the unique high quality of the positioners ensure greater precision, less ambiguity and more stability in alignment than any other instrument. In addition all optical components are mounted on a one-piece standard optical breadboard which maximizes tolerance to thermal changes and vibration.
The three laser beams intersect the sample fluid stream one below the other at equal separations. The resulting pulses from each beam are therefore separated in time and in space. The detection electronics makes use of the time separation to better resolve signals from different beams. Separation in space allows the three collected beams to be directed through individual filtering pinholes located at the focus of the objective, and then separated by two prisms into three orthogonal detection pathways. At the entry to each pathway, a collimating lens parallelizes the collected light, thereby reducing the positional sensitivity of the photomultiplier detectors and ensuring that all signal light intersects each optical filter in the collection pathway orthogonally. The result is higher signal-to-noise ratio by reduction of reflections, and increased immunity to other optical noise sources.
The measured sensitivity, which is a measure of the efficiency of the collection optics, is less than 400 MESF in the first detector channel. No other jet-in-air instrument has yet achieved sensitivity better than 700 MESF.
Speed of signal capture is essential to accurate processing of pulses (whether peak height, integrated or log amplified) detected from cells traversing the flow cytometer at high rates. Fast signal capture requires high bandwidth in the analog front end, rapid peak detection, high speed analog-to-digital (ADC) conversion and rapid recovery to receive the next pulse. The time taken to complete all the above sequence is the electronic dead time of the instrument. MoFlo has a dead time of 5.5 microseconds, shorter than any previous commercial instrument by a factor of at least three. The continuous throughput analysis capability of the machine is therefore more than 170,000 events per second. DC restoration, which is the means by which the instrument recovers from successive closely spaced pulses, ensures that accuracy is preserved down to the lowest decade even after a series of five consecutive pulses.
When the sample passes through multiple laser beams, the data from each cell is a collection of many pulses occurring at two or more moments in time. In all other instruments, the values of all the pulses from each cell must be correlated together by the instrument and passed to the computer and/or the sort decision-making electronics before the next cell can be processed. In MoFlo, however, all pulses derived from each beam in sequence are digitized and stored in a first-in-first-out buffer (FIFO) in 5.5 microseconds, and another cell intersecting the beam can be processed while the previous cell passes on to the next beam. This means that, alone among all sorters, MoFlo's dead time is 5.5 microseconds regardless of the number of beams in use.
Correlation errors in a flow cytometer are mistakes where the scatter or fluorescent properties of one cell are associated with another cell as a result of confusion in the signal processing electronics. Correlation errors are most likely when multiple laser beams are used because of mistakes associating the signals from the different beams, especially when more than one cell transits the beams at one time. Some instruments are known to have correlation errors on the order of 1 in 1000, which can pose a major limitation in the analysis and sorting of rare cells (at frequencies of 1 in 10,000 or less).
The parallel processing technique described in the Stokdijk and van den Engh paper and employed in MoFlo guarantees that correlation errors occur with a frequency of less than 1 in 1 billion particles. MoFlo is the only instrument on the market for which the correlation error rate is published and guaranteed.
MoFlo is equipped with 8 (or more by request) 256 by 256 lookup tables, into which the regions defined for sorting by the operator on the computer screen are downloaded when a sort is commenced. The lookup tables permit accurate matching of populations which form almost any shape on one or more bivariate histograms, in contrast to other instruments which use simple analog comparators for the sake of speed. On MoFlo, the lookup tables add no time to the dead time. Therefore, sorting can proceed at the same very high rate no matter how complex the shape of the sort regions or how many regions are involved in the sort decision.
At least one of the two major competitors cannot make use of non-rectangular regions without dramatic increase in the deadtime. The non-rectangular region option is also a high-priced optional extra on that instrument.
Three distinct types of sorting by flow cytometer are common, distinguished mainly by how coincident particles are handled: sorting where the yield of cells is the primary concern, outweighing purity; sorting where purity is a major concern but maximizing yield is very desirable; and sorting for the maximum achievable purity, regardless of the cost in yield. We refer to the first of these as enrich-mode sorting, to the second as purity-mode sorting and to the third as single-mode sorting.
In enrich mode, each detected target particle is sorted whether or not another particle is known to be close enough to be sorted along with the target.
In purity mode, target particles are sorted only if no "hard" coincident particle is detected (the electronics cannot tell whether the coincident particle is another target or a contaminant) and if no known contaminant particle is included in the droplet or droplets sorted. In purity mode the instrument may sort one or more droplets even when a coincidence causes a partial abort of the droplet envelope containing the target, and the presence of additional targets in the same droplets does not result in an abort. Thus, an attempt is made to maximize yield even while purity is maintained.
In single mode, the presence of any other particle, contaminant or target, in a three-drop envelope surrounding the target particle causes the sort to be aborted. This mode is most suitable for sorting into cloning trays, or where purity is paramount, or where it is essential that each sort decision takes with it one and only one target particle.
In each of the three coincidence-handling modes, there are five different ways to sort the target:
 one drop deflected, only if the particle is centered in the drop,
one drop deflected, only if the particle is centered in the drop,
 one drop deflected, wherever the particle is in the drop,
one drop deflected, wherever the particle is in the drop,
 one-or-two drops deflected; one if the particle is centered and two if it is close enough to the edge that it might drift into the next drop,
one-or-two drops deflected; one if the particle is centered and two if it is close enough to the edge that it might drift into the next drop,
 always two drops deflected (one ahead or behind, depending on phase in the drop),
always two drops deflected (one ahead or behind, depending on phase in the drop),
 always three drops deflected.
always three drops deflected.
MoFlo thus provides a total of 15 different sorting modes, each resulting in a slightly different combination of purity and yield in the sorted sample. Best yield regardless of purity is obtained with enrich-mode three-drop sorting. Best yield at high purity is obtained in purity mode, one-or-two drops, unless the event rate is close to the droplet formation frequency. Best purity at any cost is obtained in single-mode one-drop centered.
In the Cytomation Sort Unit (CSU), all controls are by optical-encoding potentiometers which provide smooth analog feel at the same time as low noise and digital repeatability. And the settings of all controls are saved in the software protocol for easy and exact reproduction of setup from day to day.
Exquisite control over all variables in the sorting system permits selection of settings for optimum performance:
 control of droplet formation frequency to 10Hz resolution and .002Hz accuracy allows precise matching of the frequency to the resonances of the flow cell and fluid system, which gives more consistent droplet formation, better stability, shorter breakoff distances and more accurate knowledge of the phase of the particle in the drop;
control of droplet formation frequency to 10Hz resolution and .002Hz accuracy allows precise matching of the frequency to the resonances of the flow cell and fluid system, which gives more consistent droplet formation, better stability, shorter breakoff distances and more accurate knowledge of the phase of the particle in the drop;
 droplet drive amplitude is variable to a resolution of 0.01 volt, which allows fine variation of the position of droplet breakoff;
droplet drive amplitude is variable to a resolution of 0.01 volt, which allows fine variation of the position of droplet breakoff;
 charge phase is variable to a resolution of 1.4 degrees (through the range of 360 degrees) - the result is one full drop tolerance to variations in drop formation, tight side-streams, small parasitic side-streams, better yield and lower contamination by virtue of more uniform charging on the sorted droplets;
charge phase is variable to a resolution of 1.4 degrees (through the range of 360 degrees) - the result is one full drop tolerance to variations in drop formation, tight side-streams, small parasitic side-streams, better yield and lower contamination by virtue of more uniform charging on the sorted droplets;
 side-stream deflection is variable in 1 percent increments to facilitate accurate positioning of the deflection streams, particularly during cloning - the result is higher yield and lower contamination;
side-stream deflection is variable in 1 percent increments to facilitate accurate positioning of the deflection streams, particularly during cloning - the result is higher yield and lower contamination;
 pulse shaping by application of a time-constant of decay is variable in 10 nanosecond increments, allowing extremely fine control of parasitic side-streams which cause contamination of sorts;
pulse shaping by application of a time-constant of decay is variable in 10 nanosecond increments, allowing extremely fine control of parasitic side-streams which cause contamination of sorts;
 droplet breakoff visualization is achieved through an infra-red video camera and display on the computer screen or on a separate monitor. The strobe which illuminates the breakoff position may be set to any phase with respect to droplet formation or charging, or may be run with slowly changing phase which shows a slow-motion droplet breakoff "movie". The display is extremely sensitive to small changes in droplet breakoff which affect sort quality.
droplet breakoff visualization is achieved through an infra-red video camera and display on the computer screen or on a separate monitor. The strobe which illuminates the breakoff position may be set to any phase with respect to droplet formation or charging, or may be run with slowly changing phase which shows a slow-motion droplet breakoff "movie". The display is extremely sensitive to small changes in droplet breakoff which affect sort quality.
Because stability of droplet formation and the distance to breakoff is critical to sort performance, the MoFlo droplet formation electronics is engineered to standards previously unknown in this industry. The droplet formation oscillator is a Direct Digitally Synthesized (DDS) sinusoid with stability of less than 5 parts per million for the period of a normal sort time and resolution of .002 Hz up to 200KHz. The DDS oscillator is also easily programmed for non-sinusoidal waveform generation when research currently under way indicates the optimum waveform for droplet formation.
As a result of the extremely high stability and resolution of the DDS oscillator, droplet breakoff is measurable in sixteenths of a drop up to 120KHz and in eighths of a drop at higher frequencies.
Because the electronic dead-time of MoFlo is 5.5 microseconds, the fundamental limitation on the number of particles per second which can be separated with high purity is the rate of droplet formation. The effective dead-time is the period of the droplet formation, which in conventional sorters is 30 microseconds or more. Higher frequency droplet formation produces a shorter effective dead-time. But higher frequency requires higher operating pressure in the sheath and sample fluid systems.
MoFlo is constructed to operate at pressures up to 100 PSI, and to withstand overload pressures up to 200 PSI. All fluidic tubing is HPLC-type high pressure material, and all valves and fittings are selected to meet the above specification.
At the time of writing the normal operating pressure is 60 PSI, which corresponds to droplet formation at 100KHz in a 70 micron nozzle. Testing is under way to confirm operation (and cell viability) at 100 PSI, which will allow 150KHz droplet rates and even higher sort rates without loss of purity or yield.
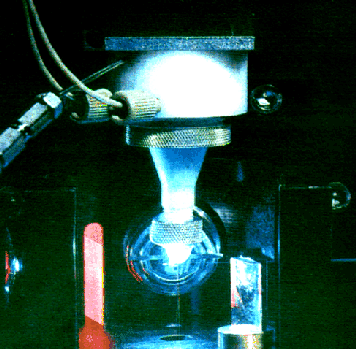
MoFlo is equipped with the most innovative jet-in-air flow cell and nozzle in flow cytometry, the Cytomation-designed and patented CytoNozzle. Nozzle tips are available in 50 micron, 70 micron, 100 micron and 200 micron diameters. The CytoNozzle patent includes 80 claims. Its key features and benefits are:
 operating and withstand pressures of 100 PSI and 200 PSI respectively;
operating and withstand pressures of 100 PSI and 200 PSI respectively;
 closely coupled piezo-electric crystal for more efficient, lower distortion droplet formation;
closely coupled piezo-electric crystal for more efficient, lower distortion droplet formation;
 tapered flow tip design which allows the laser intercept to be closer to the jet orifice and decreases coupling of stream oscillations into scatter and fluorescence signals;
tapered flow tip design which allows the laser intercept to be closer to the jet orifice and decreases coupling of stream oscillations into scatter and fluorescence signals;
 dual sheath inlet ports with independent sheath/vacuum control for more effective clearance of air-bubbles in the flow cell body;
dual sheath inlet ports with independent sheath/vacuum control for more effective clearance of air-bubbles in the flow cell body;
 the three-piece, O-ring design with minimum cavities for accumulation of particles and bacteria;
the three-piece, O-ring design with minimum cavities for accumulation of particles and bacteria;
 gently tapered conical entry to the jet smooths particle acceleration and enhances cell survivability.
gently tapered conical entry to the jet smooths particle acceleration and enhances cell survivability.
 |
 |
 |
 |