


David J. Mason and Vanya A. Gant
Division of Infection and Immunity, Department of Microbiology,
United Medical and Dental Schools of Guy's and St.Thomas's Hospitals,
London SE1 7EH, UK
 email: d.mason@umds.ac.uk
email: d.mason@umds.ac.uk
 email: v.gant@umds.ac.uk
email: v.gant@umds.ac.uk
Use of flow cytometry to investigate morphological and
physiological properties of individual organisms within bacterial
populations is becoming increasingly popular. The technique is
an excellent tool for analyzing microbial responses to
antibiotics. Antibiotic-induced changes in specific
physiological functions of the cell of interest can be probed
with selected fluorescent dyes. One such dye, bis-(1,3-
dibutylbarbituric acid) trimethine oxonol (DiBAC4(3)), a
lipophilic anion, is sensitive to changes in membrane potential.
It will enter into eukaryotic membranes only if their membrane
potential has collapsed (Wilson and Chused, 1985). Mason et al.,
(1994) extended the use of this dye to prokaryotes and have
demonstrated its application to the rapid detection of antibiotic
susceptibility. Damage to membrane integrity can be monitored
using propidium iodide (PI). This small cationic molecule binds
to nucleic acids provided it has access to them through a damaged
cell membrane. These properties have been exploited to detect
antibiotic-induced membrane damage (Gant et al., 1993; Mason et
al., 1995).
In this paper we demonstrate the use of these two
fluorescent dyes and flow cytometry in monitoring changes in
bacterial membrane potential or integrity induced by the
quinolone antibiotic (DNA-gyrase inhibitor), ciprofloxacin.
Bacterial strains, media and antibiotic. The bacterial strains
used were Escherichia coli KL16 and a clinical isolate of
Haemophilus influenzae. Both species were cultured in Iso-
Sensitest broth (filtered through a 0.2um-pore-size filter for
flow cytometry) at 37 C. For growth of H. influenzae the broth
was supplemented with Filde's extract (1%) and NADH (10mg/l).
Ciprofloxacin was a gift from Bayer (U.K.); the compound was
initially dissolved in 0.01M NaOH and then diluted to required
concentrations in distilled water.
Susceptibility testing. The minimum inhibitory concentration
(MIC) of ciprofloxacin was determined by using a standard broth
dilution method and Iso-Sensitest broth (Stokes and Ridgway,
1987). The ciprofloxacin MIC for E. coli KL16 and H. influenzae
was 0.06ug/ml.
Experimental design. Both species were grown to early
logarithmic phase (107CFU/ml) in broth culture. Cultures were
divided into three equal volumes (10ml); sparfloxacin was added
to two of the cultures at 1 and 100 times the MIC. The third
culture was retained as a control, and all cultures were
incubated for 120min. A sample (1ml) was removed from the
control culture prior to incubation, and further 1ml volumes were
removed from all cultures after 30, 60, 90 and 120 min. Each
sample was spun for 1min in an Eppendorf centrifuge at 13,000rpm,
and the pellet washed in broth once and finally resuspended in
fresh broth. Two aliquots (0.2ml) were removed from each sample
and stained with DiBAC4(3) and PI.
Bacterial staining. DiBAC4(3) (excitation 493nm, emission 516nm)
and PI (excitation 536nm, emission 617nm) were supplied by
Molecular Probes Inc., U.S.A and Sigma Chemical Co., U.K.
respectively. DiBAC4(3) was dissolved in acetone to give a stock
solution of 1mg/l. This was diluted 1:10 in 70% ethanol to give
a working solution of 100 ug/ml. PI was dissolved in deionised
water to a concentration of 100 ug/ml. DiBAC4(3) or PI were added
to 0.2ml of cell suspensions to give a final concentration of
10 ug/ml.
Flow cytometric analysis. Flow cytometric analysis was carried
out using a Bryte HS (Bio-Rad, U.K.) dual parameter flow
cytometer fitted with a mercury-xenon-arc lamp. The instrument
used a jet-over-open-surface flow cell configuration, and was
equipped with two light scatter detectors (greater than 15 degrees
and less than 15 degrees) and two
fluorescence detectors (beam split at 520 nm). Fluorescence
detection (gated by light scatter parameters) was carried out
using a FITC filter block with the following characteristics:
excitation, 470-490 nm; band stop, 510 nm; and emission greater than 520nm.
All detectors were used with logarithmic amplification; sample
flow and sheath pressure were set to 2 ul/min and 0.7 Bar
respectively.
In the following experimental results the proportion of cells in
the bacterial population which exhibited dye-associated
fluorescence (and therefore had depolarised cell membranes or
damaged membrane integrity depending on the dye) is expressed as
a percentage.
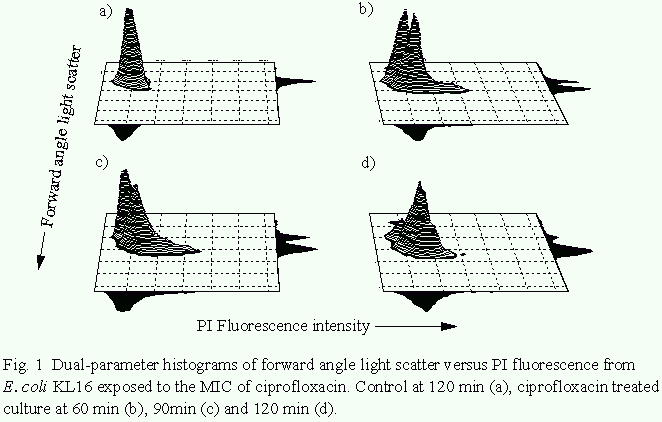
The effects of ciprofloxacin action on E. coli KL16. Fig. 1
shows dual parameter histograms of forward angle light scatter
versus PI fluorescence for a culture of E. coli KL16 exposed to
ciprofloxacin at the MIC for 120 min. Forward angle light
scattered by organisms is seen to increase over 120 min (similar
results were obtained with H. influenzae). At 100xMIC, however,
no increase in forward angle light scatter was observed from
either bacterial species. Propidium iodide fluorescence at the
MIC increased slowly over 120 min, with 15% of organisms being
rendered fluorescent after 120min. In contrast, the proportion
of cells from a control culture exhibiting propidium iodide-
asscociated fluorescence after 120min was 1.5%. Propidium iodide
uptake following exposure to100xMIC of ciprofloxacin for 120 min
was similar to that seen at the MIC.
The proportion of cells exhibiting DiBAC4(3)-associated
fluorescence following exposure to the MIC of ciprofloxacin
reached 20% after 120min. This represented a minor increase over
the control value (12%), but did not reach statistical
significance at the 5% level (Mann-Whitney U test). In contrast,
exposure to 100xMIC for 120min resulted in 95% of the
population exhibiting DiBAC4(3)-associated fluorescence (Mason et
al., 1995).
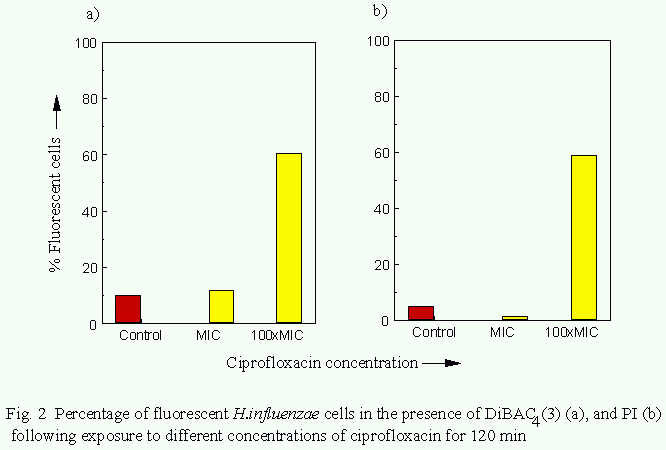
The effects of ciprofloxacin action on Haemophilus influenzae.
In cultures exposed to ciprofloxacin at the MIC the proportion
of organisms exhbiting DiBAC4(3)- or PI-associated fluorescence
after 120 min was 7% and 1% respectively. Following exposure to
100xMIC for 120min the proportion of organisms stained with
DiBAC4(3) or PI increased to 54% and 28% respectively (Fig. 2).
The proportion of organisms from control cultures rendered
fluorescent by DiBAC4(3) or PI was approximately 10% and 5% respectively.
The results illustrate how flow cytometry can be used to detect
heterogeneity wthin a microbial population in terms of
susceptibility to an antibiotic. Other techniques for
susceptibility testing such as the disc diffusion assay or the
broth dilution method, consider the bacterial population as a
whole and any heterogeneity in response to an antibiotic within
this goes un-detected.
In addition, detection of membrane damage using the
fluorescent physiological probes demonstrates how flow cytometry
can be used to provide information on the mechanism of
antimicrobial activity of antibiotics. The extent of membrane
damage induced by ciprofloxacin was shown to increase with
antibiotic concentration in both bacterial species. This
concentration-dependent effect may be due to ciprofloxacin
complexing with membrane stabilizing cations (Chapman and
Georgopapadakou, 1988; Smith, 1990). Changes in forward angle
light scatter profiles upon exposure to ciprofloxacin at the MIC
represent filamentation of the organisms (confirmed by phase
contrast microscopy). For a more detailed discussion of how
these results relate to the mechanism of action of this
antibiotic see Mason et al., (1995).
The authors wish to thank Rhône D. P. C. Europe and the Trustees
of St Thomas' Hospital for their financial support.
Chapman, J. S., and N. H. Georgopapadakou. 1988. Routes of
quinolone permeation in Escherichia coli. Antimicrob. Agents
Chemother. 32:438-442.
Gant, V. A., G. Warnes, I. Phillips, and G. F. Savidge. 1993.
The application of flow cytometry to the study of bacterial
responses to antibiotics. J. Med. Microbiol. 39:147-154.
Mason, D. J., R. Allman, J. M. Stark and D. Lloyd. 1994. Rapid
estimation of bacterial antibiotic susceptibility. J. Microscopy
176:8-16.
Mason, D. J., E. G. M. Power, H. Talsania, I. Phillips and V. A.
Gant. 1995. Antibacterial action of ciprofloxacin. Antimicrob.
Agents Chemother. 39:2752-2758.
Smith, J. T. 1990. Effects of physiological cation concentration
on 4-Quinolone absorbtion and potency, p.15-21. In G. C.
Crumplin (ed), The 4-Quinolones Antibacterial Agents in Vitro.
Springer-Verlag, London.
Stokes, E. J., and G. L. Ridgway. 1987. Clinical microbiology.
Edward Arnold Publishers Ltd, London.
Wilson, H. A., and T. M. Chused. 1985. Lymphocyte membrane
potential and Ca2+ sensitive potassium channels described by
oxonol dye fluorescence measurements. J. Cellular Physiol. 125:72-
81.
 Back to Flow Cytometry and Microbiology Introductory Page
Back to Flow Cytometry and Microbiology Introductory Page
