



FLOW CYTOMETRY IN
OCEANOGRAPHY
Dominique Marie, Frédéric Partensky and Daniel Vaulot
CNRS et Université Pierre et Marie Curie, Station Biologique, BP 74, 29680 Roscoff, France
In the last decade, flow cytometry (FCM) has been increasingly used to analyze natural communities of marine microorganisms. FCM provides rapid and accurate measurements of individual phytoplanktonic cells that are too dim to be discriminated by epifluorescence microscopy. It allows the discrimination between auto- and heterotrophic populations as well as between cells and detritus or suspended sediments. FCM is particularly well suited for the study of the smallest size class of the plankton (below 2 µm), called picoplankton and that is composed by 4 major groups: heterotrophic prokaryotes, prochlorophytes (Prochlorococcus, Chisholm et al., 1992), cyanobacteria (Synechococcus, Waterbury et al., 1979) and eukaryotes. These small organisms dominate the biomass in the open ocean, reaching respective concentration ranges of 106 - 105, 105 - 103, 105 - 103 and 104 - 102 cells per ml.
Abundance of Phytoplanktonic Populations
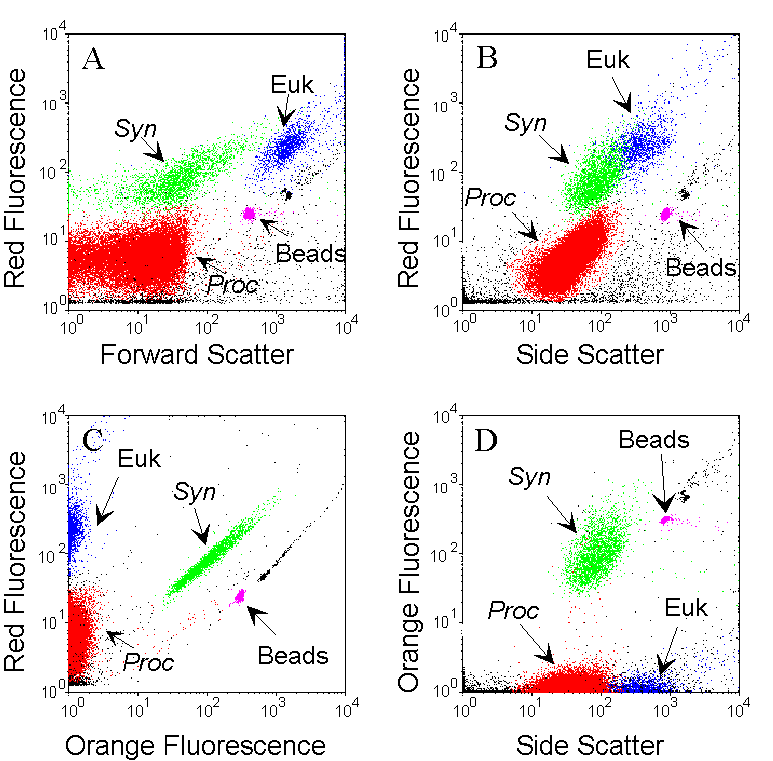
The combined analysis of the light-scattering parameters and of the fluorescence of natural photosynthetic pigments (chlorophyll, phycoerythrin) allows the identification of different groups that differ in terms of size and pigment contents. Example cytograms were obtained for one sample collected at 15 m, in oligotrophic waters (Pacific Ocean) containing Prochlorococcus (Proc, red), Synechococcus (Syn, green) and picoeukaryotes (Euk, blue). Data acquisition is triggered by red fluorescence to reduce interference’s from non fluorescent particles. Subpopulations are interactively defined with gates and identified by the combination of all recorded parameters. The Synechococcus population is discriminated from other phytoplankters by its orange fluorescence (due to the presence of phycoerythrin) on the orange versus red fluorescences cytogram (C). Prochlorococcus cells that are smaller and less fluorescent are distinguished from picoeukaryotes on the bivariate distribution of the Forward and Side Scatters (as a function of size) versus red fluorescence (A and B). Fluorescent microspheres (0.95 µm beads) are added as internal reference. All other particles are non photosynthetic detrital particles.
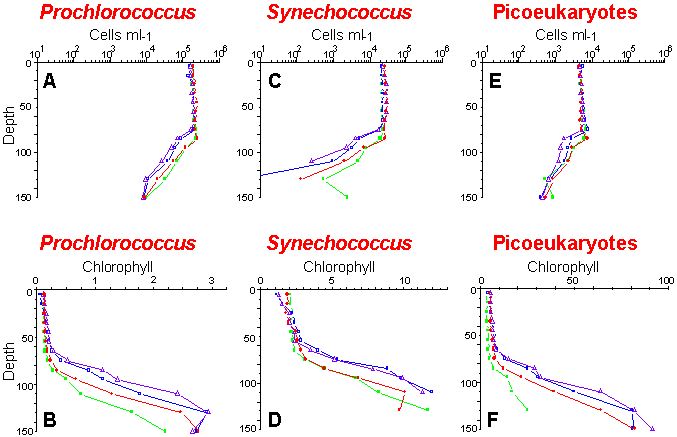
Vertical distributions of cell concentrations and chlorophyll per cell for three picoplankton populations: Prochlorococcus (A and B), Synechococcus (C and D) and picoeukaryotes (E and F). Samples were collected and analyzed on board ship during the OLIPAC cruise at different times of the day: 8:00 (green), 14:00 (blue), 18:00 (violet) and 22:00 (red).
DNA, Cell Cycle and Bacterial counts
Nucleic acid stains provide information on the cell cycle distribution of photosynthetic prokaryotes, from which in situ growth rates may be estimated.
After staining with a DNA-specific dye, cells are analyzed by flow cytometry. Each cell has an intensity of fluorescence that is assumed to be proportional to its DNA content. Under normal conditions, all non-replicating cells should have the same DNA content, usually expressed as 2C for the eukaryotes or as 1C for the prokaryotes that corresponds to the G0/G1 phase. When cells begin to synthesize DNA, they enter into the S phase until they have a DNA content equal to the double of their initial set. This corresponds to the G2 phase.
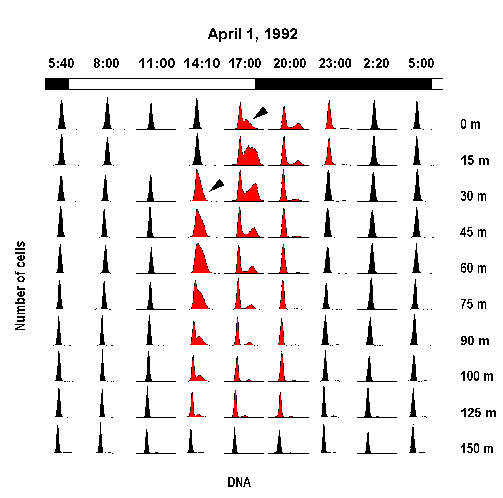
Change in Prochlorococcus cell cycle distribution for a depth profile in the equator (140°W) during one diel cycle (1 April 1992). Arrow-heads point to the initiation of the S phase (Vaulot et al. Science, 1995).
Flow cytometric analysis of bacteria, that have generally a very low DNA content and that have no pigments, requires the combination of highly fluorescent stains and sensitive instruments. Initially the UV-excited dyes DAPI or Hoechst 33342 have been used for this purpose. Recently, blue-excited dyes such as YOYO-1, PicoGreen or SYBRTM Green-I have been introduced that make this type of analysis possible on small low-cost flow cytometers (laser line = 488 nm).
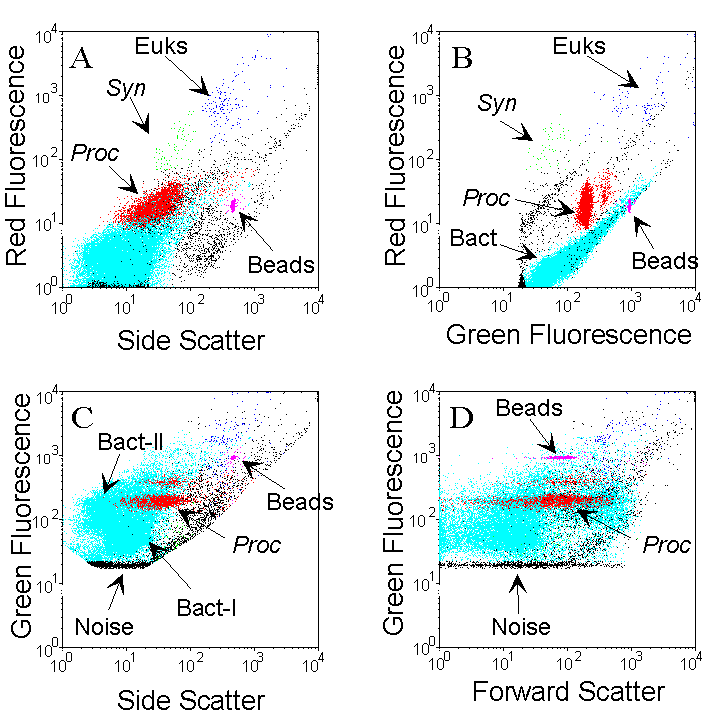
The different populations are identified by the combined analysis of light-scattering parameters, fluorescence of photosynthetic pigments (chlorophyll, phycoerythrin) and fluorescence of the complex DNA-dye. Example cytograms were obtained for one sample collected at 95 m, in oligotrophic waters (Pacific Ocean) containing Prochlorococcus (Proc, red), Synechococcus (Syn, green), picoeukaryotes (Euk, blue) and heterotrophic bacteria (.cyan). Two populations of discriminated on the Side scatter vs. Green fluorescence cytogram. Fluorescent microspheres (0.95 µm beads) are added as an internal reference. bacteria, called Bact-I and Bact-II, can be discriminated on the Side scatter vs. Green fluorescence cytogram. Fluorescent microspheres (0.95 µm beads) are added as an internal reference.
Molecular probes (18s rRNA)
The taxonomy of small eukaryotic picoplankton is still poorly known nowadays. Most species have very few morphological features and can hardly be discriminated, even at the class level, by classical methods such as optical microscopy. Fluorescent oligonucleotide probes targeted to 18S rRNA appear as very promising tools for this purpose, allowing the identification of specific groups within complex communities.
From knowledge of the ribosomal DNA sequences of microorganisms, nucleic acid probes specific for taxa can be designed. They are complementary to a region of the ribosomal RNA molecule which is unique to the target group and can be used as "phylogenetic stains" once they have been labeled with a fluorochrome. Oligonucleotide probes will hybridize to their homologous strand on the rRNA molecule within preserved cells. Labeled cells are detected by the probe-conferred fluorescence. This method is now commonly used for the identification of bacteria, but can also be used for the detection and identification of phytoplankton. Such probes are especially useful for the study of the smallest algae (picoeukaryotes) for which identification requires much time and expertise using traditional techniques because morphological characters are not readily available. Routinely used on cultured species, whole-cell hybridization has yet to be applied to natural samples where the cell population of interest (a given species or genus for example) is part of a complex community.
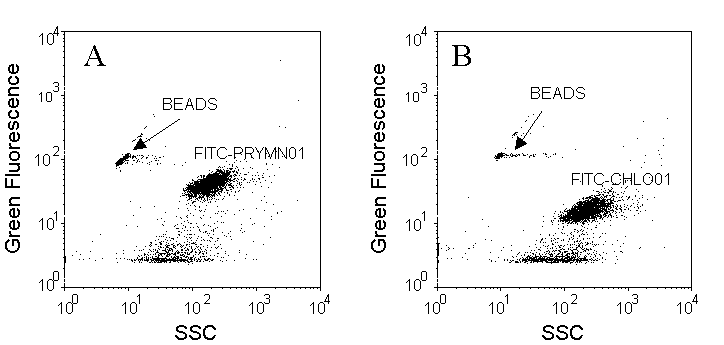
Cells are discriminated on SSC versus green fluorescence (FITC) cytograms. Pleurochrysis carterae (Prymnesiophyceae) has been chosen as an example (A and B) for in situ hybridization with a probe specific for the Non-chlorophyte (FITC-PRYMN01, A) and as a control, with a probe specific for the Chlorophytes (FITC-CHLO01, B) that should not label these cells. Fluorescent microspheres (0.95 µm beads) were added as internal reference.
Selected references
Amann, R. I., Ludwig, W., Schleifer, K.-H. (1995). Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59: 143-169
Button, D. K., Robertson, B. R. (1989). Kinetics of bacterial processes in natural aquatic systems based on biomass as determined by high-resolution flow cytometry. Cytometry 10: 558-563
DeLong, E. F., Wickhan, G. S., Pace, N. R. (1989). Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science 243: 1360-1363
Lange, M., Guillou, L., Vaulot, D., Simon, N., Amann, R. I., Ludwig, W., Medlin, L. K. (1996). Identification of the class Prymnesiophyceae and the genus Phaeocystis with ribosomal RNA-targeted nucleic acid probes detected by flow cytometry. J. Phycol. 32: 858-868
Li, W. K. W., Jellett, J. F., Dickie, P. M. (1995). DNA distribution in planktonic bacteria stained with TOTO or TO-PRO. Limnol. Oceanogr. 40(8): 1485-1495
Marie, D., Partensky, F., Jacquet, S., Vaulot, D. (1997). Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Appl. Environ. Microbiol. 63: 186-193
Marie, D., Vaulot, D., Partensky, F. (1996). Application of the novel nucleic acid dyes YOYO-1, YO-PRO-1 and PicoGreen for flow cytometric analysis of marine prokaryotes. Appl. Environ. Microbiol. 62: 1649-1655
Monger, B. C., Landry, M. R. (1993). Flow cytometric analysis of marine bacteria with Hoechst 33342. Appl. Environ. Microbiol. 59: 905-911
Simon, N., Lebot, N., Marie, D., Partensky, F., Vaulot, D. (1995). Fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes to identify small phytoplankton by flow cytometry. Appl. Environ. Microbiol. 61: 2506-2513
Simon, N., Barlow, R. G., Marie, D., Partensky, F., Vaulot, D. (1994). Flow cytometry analysis of oceanic photosynthetic picoeucaryotes. J. Phycol. 30: 922-935
Vaulot, D., Marie, D., Olson, R. J., Chisholm, S. W. (1995). Growth of Prochlorococcus, a photosynthetic prokaryote, in the equatorial Pacific Ocean. Science 268: 1480-1482
Vaulot, D., Partensky, F. (1992). Cell cycle distributions of prochlorophytes in the North Western Mediterranean Sea. Deep Sea Res. 39: 727-742
Vaulot, D., Courties, C., Partensky, F. (1989). A simple method to preserve oceanic phytoplankton for flow cytometric analyses. Cytometry 10: 629-635
 Back
to Flow Cytometry and Microbiology Introductory Page
Back
to Flow Cytometry and Microbiology Introductory Page
