

One assumption of multiparameter analysis is its capacity to bring
out several parameter during the same analytic run. This would remain devoid
of any significance were it not accompanied by a faculty to go on and reprocess
the data in a multivariate statistical analysis that enables the operator
to gather information about the expression of certain parameters in subpopulations
selected on the basis of other preceding parameters. A classic practical
example of this procedure is seen in the opportunity it affords of simultaneously
obtaining data for distribution of two or more membrane antigens in cell
populations of varying sizes, distinguishable therefore on the basis of
their physical characteristics.
There is no doubt that a multiparameter approach to immunophenotyping has been made possible in recent years by a series of steps ahead in instrumentation and reagent technology. It is still particularly interesting to note that what made this approach necessary were, so to speak, a series of "cultural" factors that have modified the application of flow cytometry to laboratory diagnostics. Important to remember in this respect are the following points:
1) not using mononuclear cells obtained through density gradient, but whole lysed blood samples with its consequent need to analyse highly heterogeneous samples;
2) indicating the very low-population cell groups as in the case of the valutation of the minimal residual disease;
3) producing results with very high accuracy, as in CD4+ and CD34+ cells counts;
4) unequivocally identifying cell populations that are not defined well enough by the expression of only one antigen, as in the case with CD8+ T lymphocytes, which have to be distinguished from NK cells on the basis of simultaneous expression of CD3 antigen.
For these and other reasons, very often the multiparameter approach is the only suitable one for immunophenotyping.
Although it is possible to perform multiparameter analysis by collecting data about two scatter measurements and only one fluorescence signal, as in the case of simultaneous analysis of CD4 antigen on lymphocytes and monocytes, these days the term "multiparameter analysis" is something of a synonym for "multifluorescence" or "multicolour analysis".
From the technical side, the cardinal problem with multicolour analysis lies in getting a sufficient number of different probes excitable at the same wavelength, but able to emit in distinct spectrum areas and hence remaining distinguishable from each other (see Tab. 1).
|
|
||
|
|
|
|
| CASCADE BLU | UV (351-365 nm) | BLUE (460 nm) |
| AMCA | UV (351-365 nm) | BLUE (460 nm) |
| FITC | BLUE (488 nm) | GREEN (514 nm) |
| PHYCOERYTHRIN | BLUE (488 nm) | ORANGE (580 nm) |
| TANDEM PE-TR | BLUE (488 nm) | RED (610 nm) |
| TANDEM PE-Cy5 | BLUE (488 nm) | DEEP RED (670 nm) |
| PerCP | BLUE (488 nm) | DEEP RED (670 nm) |
| ALLOPHYCOCYANIN | RED (600-633-647 nm) | DEEP RED (660 nm) |
| CYANIN 5 | RED (633-647nm) | DEEP RED (670 nm) |
In this chapter we shall consider cytometric multifluorescence
techniques in order of complexity, with detailed attention to technical
characteristics and practical difficulties, and how to overcome them. Only
two types of flow cytometrs will be considered in that these are diffuse
in clinical and research laboratories, and that is the single laser flow
cytometer with argon laser (emission at 488 nm), and the dual-laser machine
equipped with an argon laser plus a secundary laser with red emission at
around 630 nm.
TWO-COLOUR ANALYSIS
ANALYSIS WITH A SINGLE LASER CYTOMETER
Two-colour fluorescence analysis is the simplest type of multicolour analysis and, although it is possible to carry it out with whatever couple of simultaneously excitable probes, in the overwhelming majority of cases what is used is fluorochrome Fluorescein (FITC) together with Phycoerythrin (PE) - the latter being particularly adapted to this type of work in that:
1) its absorption range shows a shoulder close to the 488 nm region which is what excites fluorescein;
2) compared to FITC, PE’s emission range presents a peak in a region far enough to permit distinct evidentiation.
Phycoerythrin also has two other highly desirable features. It is chemically a very stable molecule, very bright in that it has high quantum efficiency, easily conjugable with antibody molecules. Nevertheless, a satisfying use of the couple FITC/PE requires attention to the following:
1) FITC’s efficiency, i.e. brightness, depends on the
pH of the solution in which the cells are resuspended;
2) unlike FITC, which is a very small molecule, Phycoerythrin is a very large one, with a molecular weight of 240 kD and likely to be of sterical hindrance if used together with other bulky probes;
3) the Phycoerythrins are a family of natural dyes, slightly differentiated among themselves by different spectral features, source of production and other characteristics. This heterogeneity is better seen in commercial PEs, whose behaviour is not always reliably reproducible. Monoclonal conjugated antibodies produced by an unfamiliar company should be thoroughly tested before use.
Compensation in-two-colour analysis: theoretical and practical aspects.
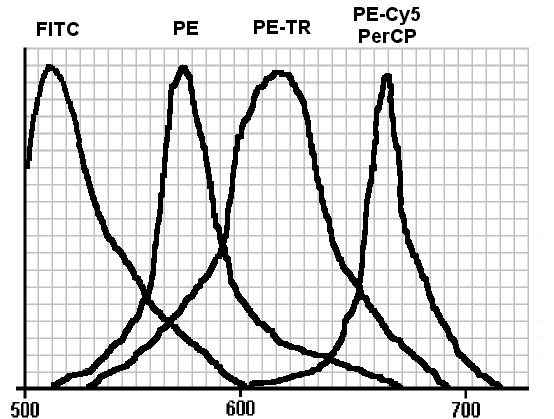
Careful observation of fig. 1 brings out some fundamental points:
1) as we have already pointed out, the emission peaks of FITC and PE are far enough from each other to allow distinct collection of both;
2) their best separation is achievable by isolating the regions of maximum emission using band-pass optical filters, actually placed in front of the photomultipliers collecting the two signals;
3) nonetheless, however narrow the ranges of filters are, complete separation of the two signals will never be possible, in that the tail of each fluorochrome’s signal overlaps the maximum emission peak of the adjoining fluorochrome.
We can now consider FITC’s behaviour in a two-colour system with a PMT1 for the green FITC signal, and a PMT2 for the PE orange one. Although in the optical microscopy FITC has a characteristic apple-green signal which is properly detected by PMT1, its emission spectrum presents an orange component, which is detected by PMT2 also.
According to this phenomenon, in a non-compensated two-colour system, a cell subset stained only with FITC behaves as if it were also stained with PE. The intensity of this unexpected signal, and consequentially the offset of the cluster from its proper position in the cytogram, are proportional to the extent of the overlap, or spillover by FITC emission into the PE region.
Likewise, a cell subset stained only with PE behaves as if it were faintly stained with FITC; since the overlap of PE on the FITC range is much lower, the offset of the cluster from its proper position is accordingly smaller.
This spillover phenomenon, also known as "leakage", not only generates the grafic artifact of cluster offsetting in the graphical representation of bivariate analysis, but it is also an effective obstacle when it comes to counting double-labelled cells. In activated T-lymphocytes for example, HLA-DR antigen is continually being expressed from a minimum to a maximum between CD3+DR- and CD3+DR+ cells and the accuracy of detection of double-labelled cells depends on the proper subtraction of CD3+DR- component. But in the graphic representation of a bivariate analysis, a subset is considered negative for an antigen only when allineated with its negative control. Such allineation is impossible in an uncompensated system and subtraction cannot be done.
By "compensation" we intend correction of the spillover between channels, a procedure made possible by the fact that, all analytical conditions being constant, a fluorochrome’s contribution to the signal detected by the PMT of the other fluorochrome is constant through the time of the analysis. Hence all that is necessary in the case of two colour FITC/PE analysis is the following:
1) to subtract an amount proportionate to the unwanted signal routed by FITC from the output of PMT2, i.e. to subtract FL1 from FL2 (FL2-FL1%);
2) to subtract an amount proportionate to the unwanted signal routed by PE from the output of PMT1, i.e. to subtract FL2 from FL1 (FL1-FL2%).
The cytometer is well compensated when the clusters in the cytogram are in their boundary limits, and that is, in the case of single-labelled cells, where the proper location of the FL1 cluster is parallel to the x axis with its leading edge aligned to the boundary limits of the negative control, whereas the location of the FL2 cluster is similar but parallel to the y axis.
Spectral compensation can be done "on-line" or "off-line". In the case of "on-line", the compensation is set before the analytical run starts. In "off-line", the sample is acquired in an uncompensated system and the results are subsequently handled by a computer programme, which will modify the parameter values in each event, to simulate the behaviour the sample would have displayed in a compensated system. "Off-line" compensation relies on suitable analysis programmes, and asks for a series of controls to which to conform when handling results.
ANALYSIS WITH A DUAL-LASER CYTOMETER
It is perfectly possible to carry out a two colour analysis with a dual laser cytometer, exciting the first fluorochrome with the first laser and the second fluorochrome with the second one. In the case of a machine equipped with a primary argon laser plus an helium-neon or a diode laser, for example, it is possible to excite the two probes Fluorescein (excitation in blue, emission in green) and Allophycocyanin (excitation in red, emission in deep red) simultaneously. The benefit from this procedure is that there is no need to perform spectral compensation since the two emissions are too far from each other to be of interference.
THREE-COLOUR ANALYSIS
ANALYSIS WITH A SINGLE-LASER CYTOMETER
Three colour analysis with a single-laser cytometer asks for a third fluorescent molecule alongside the FITC/PE couple but distinguishable from PE although also excitable in blue. Molecules with these spectrum characteristics are thin upon the ground in nature and for a long time none were commercially available.
The fluorochromes available at the moment are divisible into two main groups with the common characteristic that they can transfer light radiations along their own structure, progressively increasing them in wavelength.
The first group consists of one molecule, natural in origin, the so-called PerCP (Peridinin Chlorophyll Protein) - a pigment to be found in dynoflagellates, monocellular organisms which live at great depth under the sea. Its function is to transport energy made available by the only light that can penetrate to that depth, blue light, to the chlorophill’s chromophores, which absorb red light.
The second group comprises the so-called fluorochrome tandem, built for two fluorescent molecules with complementary spectral characteristics, using non-covalent bonds. In this tandem, the first molecule has an emission range that coincises with the second molecule’s excitation range. Because of the great proximity guaranteed by these two structures with non-covalent bonding, excitation of the former molecule is transferred directly and without emission of light to the second molecule, which emits within its own range. In other words a fluorochrome "tandem" of this kind behaves as if it were a single molecule capable of the excitation of the first half and the emission range of the second. In other words the Stokes shift of this fluorochrome is equivalent to the sum of the Stokes shift of both the molecules that go to make it up. Although various types of tandem fluorochromes have been synthesised, only two of these are in commerce. These are the Phycoerythrin and Texas Red tandem (PE-TR) and the Phycoerythrin and Cyanin 5 tandem (PE-Cy5). These two compounds differ from each other in emission range, which is around 610 nm for PE-TR and around 670 nm for PE-Cy5 (see fig. 1).
The fluorochromes described above are bright and reliable as molecules. Their efficacious use depends on observing the following basic guidelines.
1) Exposure to light or other unsuitable conditions may break the non-covalent bonding of the tandem. Should this happen, the second half of the tandem is lost and hence the presence of Phycoerytrin molecules no longer bonded to their fluorochromic partners means that these emit only in their own range. A tandem partially damaged in this way emits not only in red (FL3) but also in orange (FL2) creating artifacts that are of difficult interpretation.
2) The PE-Cy5 type of tandem can bond, aspecifically, with dead cells, with elements of the monocyte line, with sub-populations of B-lymphocytes and to acute promyelocyte leukemia (M3) cells.
3) Working with double-laser cytometers, the choice of tandem fluorochrome must be done with care, excluding those moelcules whose second half might be inappropriately excited by the actual secondary laser. For example, unless there is interlaser compensation circuiting (q.v.) the PE-Cy5 tandem can not be used in a system with a helium-neon laser.
4) The PerCP molecule is light-sensitive and can be analysed only using instruments with a low power primary laser. As an alternative, the 488 nm emitting laser should be turned down to values that do not exceed 30 mW.
Compensation in-three-colour analysis: theoretical aspects.
Observation of the spectral profile of those fluorochrome trios most often to be found in three-colour analysis with single-laser cytometers immediately shows that the introduction of a third fluorochrome complicates how compensationn is carried out. Take, for example, the FITC / PE / PE-TR system. Here, not only is there overlapping of emissions between FITC and PE, but also between PE and the PE-TR tandem. This means not just having to subtract a quota of FL2 from FL1 (FL1-%FL2) and a quota of FL1 from FL2 (FL2-%FL1) but also that of subtracting a quota of FL3 from FL2 (FL2-%FL3) and a quota of FL2 from FL3 (FL3-%FL2).
Observations of spectrum profiles also mean that:
1) measurement of the Phycoerythrin signal is the most critical in that it has to take account of the entry of fluorochrome 1 and 3 into the detector;
2) the entity of the compensation itself (FL2-%FL3) is in inverse proportion to the distance between the peaks of the second and the third fluorochrome and is therefore maximum where the PE-TR is being used and minimum with PerCP or the PE-Cy5 tandem;
3) seeing that in general there is no actual compensatory
circuiting for FL1 and FL3 spillover, the use of a bad quality fluorochrome
tandem whose PE loses signals, keeps the "on line" system non-compensable.
Compensation in-three-colour analysis: empirical approach.
From a practical point of view, compensation for three on-line fluorescences is a procedure that, for the non-expert user, can create some difficulties that are superable with method and pathience. The following is how we deal with it in our flow cytometry unit.
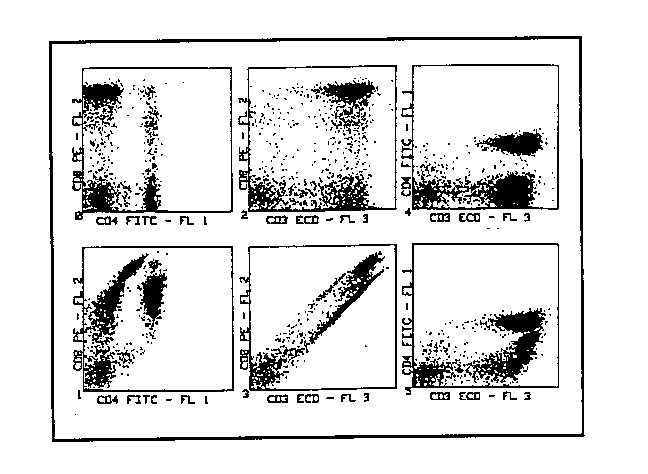
1) First of all it is necessary to build up a sufficient amount of standard to analyse, for example a sample of whole blood, stained with antibodies conjugated with fluorochromes for which to establish what the compensation is. The example proposed has been carried out with an anti-CD3 antibody conjugated with PE-TR tandem, an anti-CD4 antibody conjugated with FITC, and an anti-CD8 antibody conjugated with PE. Analysis was confined to the lymphocytes, gating on physical parameters.
2) We begin by observing the cytogrammes produced by the
analysis done with no compensation and compare these to the cytogrammes
"as they ought to be" (fig. 2). The model or control analysis can be represented
by a series of two-colour analyses, taken one alongside the other and involving
the three parameters explored during three-colour analysis.
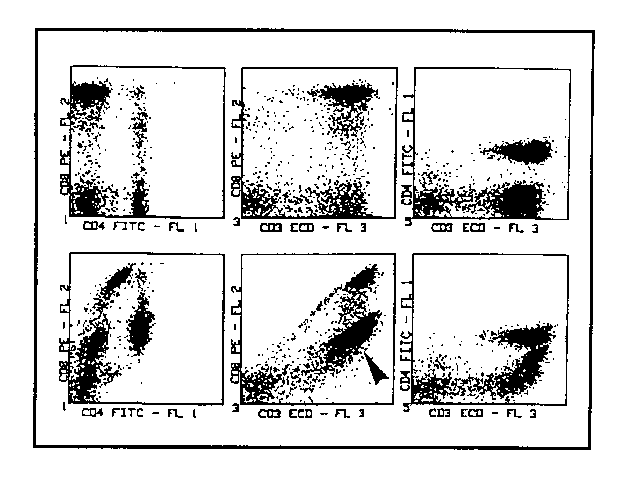
3) Given that the "critical" fluorescence in this system is FL2, we start by subtracting FL3 from it, i.e. working on (FL2-%FL3) in the intention of lowering the CD3+CD8- lymphocyte cluster to the point of aligning it with the cluster of negative lymphocytes. This is an operation to be done in real time, observing FL2 vs FL3 cytogramme. Offsetting of the CD3+CD8- cluster from its proper position not only depends onthe FL3 spillover but also on that of FL1. Hence it will not be possible to arrive at complete correction by working only with (FL2-%FL3), and for the moment we shall satisfy ourselves with a partial one. In the example, the value of (FL2-%FL3) has been set at 50% and it is evident that the CD3+CD8- cluster is approaching the correct position but is not there yet. The correction of the offset of positive elements for FL2 has also become evident in the cytogramme FL1 vs FL2 where the CD4+CD8- elements are also tending towards the proper position of alignement to the double negative lymphocyte cluster (fig. 3). The apparent paradox that subtraction of the inappropriate contribution of FL3 should modify the position of the elements positive only for FL1 in the FL1 vs FL2 cytogramme is explained by the fact that the CD4+ elements are nonetheless positive for CD3. The staining with the PE-TR conjugated anti-CD3 MoAb gives them a red signal that is sufficient to make them detectable by FL2 photomultiplier.
4) Having carried out the first partial correction of the CD3+CD8- cluster position working with (FL2-%FL3), the operation is completed using (FL2-%FL1) until such time as the expected position is reached (fig. 4). Please note that the correct positioning of the CD3+CD8- cluster is not tied up to an unique pair of compensation values but obtainable by imposing a series of varied and complementary pairs of values of (FL2-%FL3) and (FL2-%FL1).
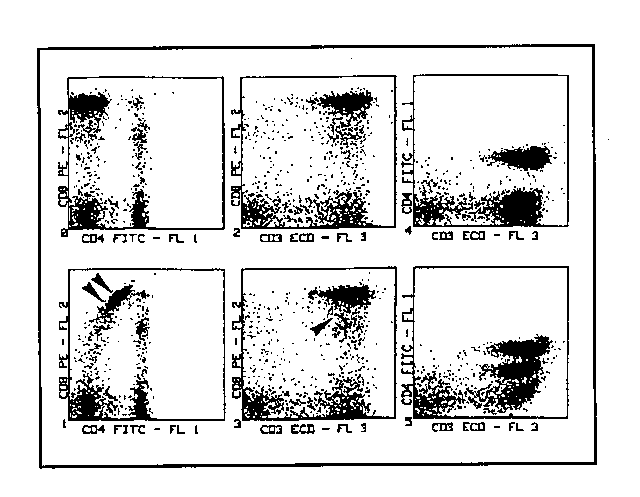
5) Here we move on to FL3 compensation. The outgoing PMT3/FL3 signal has a percentage subtracted from it, proportional to the "spurious" one produced by PE. The system will be well compensated when the CD3+CD8+ cluster in the cytogramme is what is to be expected, i.e. the characteristic one for double-marked elements in the right-upper quadrant (fig. 5).
6) Finally we pass on to compensate FL1, subtracting from the outgoing PMT1/FL1 signal a percentage proportionate to the "spurious" signal produced by PE. Compensation may be considered correct when the CD8+CD4- cluster in the cytogramme is what is to be expected, that is in alignement with the double negative lymphocyte cluster in the left-lower quadrant. At this point, the system is compensated and the compensation values thus obtained can be entered into the cytometer computer for further use in case of necessity.
Compensation in-three-colour analysis: final considerations.
Compensation values obtained using the above procedure are obviously valid for that particular run of analysis and are liable to modify with variation in critical parameters like, e.g., the spectrum characteristics of the fluorochrome used, the power of the light sources involved, the setting of the photomultipliers, or, worse, varying in intra-series analysis conditions such as density of expression of antigens under examination or possibility of extinction between too close complementary fluorochromes. The greater variations do call for a new equilibrium, the lesser ones can often be put right with minor adjustments done in real time.
ANALYSIS WITH A DUAL-LASER CYTOMETER
In the form of dual laser we are dealing with, i.e. argon and helium-neon or diode, usually it is the primary laser to run the FITC/PE couple and the secondary one looks after the third fluorochrome, Allophycocianin (APC) or Cyanin 5 (Cy5). If the laser beams are spatially separated, compensation work is greatly facilitated, since the cell marked with antibodies conjugated with fluorochromes moves first of all across the primary laser beam and only then - and in typical cases after 10-15 m s - crosses the secondary one’s. This involves a cell, marked with the trio FITC/PE/APC, emitting the APC red signal at a moment and point in the optical run, different from where and when it was emitting the orange and green signals of FITC and PE. Separating out like this, over time and space, makes compensation work between FL3 and other fluorescences superfluous to the point of making simultaneous analysis of similar emission range fluorochrome possible, and this because each is being piloted by a different laser. As a result, in a two laser, three-colour analysis such as the one we have disceussed here, all that will be required is to compensate between FL1 and FL2, without bothering about FL3 compensation.
FOUR-COLOUR ANALYSIS
ANALYSIS WITH A SINGLE-LASER CYTOMETER
Four-colour analysis with a single argon laser cytometer is possible using simultaneous marking with FITC, PE and two tandems, PE-TR and PE-Cy5. Analysis of spectrum profiles clearly demonstrates that the peaks can be sampled separately. In a single laser cytometer, the four different signals stem simultaneously at the same moment and in the same point. The work involved is to separate them out into their various emissions and this is the exclusive job of the dichroic mirrors and the band-pass filters. On line compensation is thus made a very critical executive part of operation. As happens in three-colour analysis, also in that with four fluorescences it is necessary to compensate as follows between the various pairs pairs FL1 & FL2 (FL1-%FL2 and FL2-%FL1), FL2 & FL3 (FL2-%FL3 and FL3-%FL2), and FL3 & FL4 (FL3-%FL4 and FL4-%FL3).
ANALYSIS WITH A DUAL-LASER CYTOMETER
When a dual laser is used for four colour analysis the primary laser usually takes on the work with the FITC, PE and PE-TR or PerCP fluorochromes and the secondary laser deals with APC or Cy5, or another fluorochrome such as red-excitable DNA+probe such as ToPro3. As with three-colour analysis, the chance of separating up time and space makes compensation work between FL4 and other fluorescences unnecessary to the point that in the present example all that is required is to compensate between FL1 & FL2 (FL1-%FL2 and FL2-%FL1) and FL2 & FL3 (FL2-%FL3 e FL3-%FL2), without having to worry about compensating between FL4 and other fluorescences.
There is a disadvantage though, with the parallel beam lay-out: it finds the PE-Cy5 tandem hard to deal with. This tandem, in fact, emits an earlier "appropriate" signal when excited by the primary laser but also a secondary and "inappropriate" one ehen it crosses the secondary laser’s beam. This "inappropriate" signal comes from the fact that the other half of the tandem, namely Cyanin 5, is excitable at 633 nm. This inconvenience may be obviated with an intelaser compensation which subtracts a signal proportinated to that of Cy5 in FL3 from FL4.
ANALYSIS WITH MORE THAN FOUR-COLOUR
Multicolour analyses with more than four fluorochromes are not impossible but they do ask for an instrumental architecture larger than that discussed here. The fifth fluorochrome is usually one excitable by UV, like Cascade Blue (CB) or an amino-methyl cumarin (AMCA) derivate. This approach is often chosen because there are argon lasers that can emit a line in the UV simultaneous to the line at 488 nm. Theoretically, it is also possible to put a tandem molecule like APC-Cy7, able to emit in infra-red, with the first half of tandem run by the secondary laser together with APC. This tandem, known also as Allo-7, is not yet commercially available but it looks as if it will have some interesting potential uses, like the following:
1) a four-fluorescence / dual laser system, where the primary laser excites FITC and PE and the secondary one APC and Allo-7, with a drastic drop in the necessity for compensation between the pairs FL1&FL2 and FL3&FL4;
2) a seven-fluorescence / dual laser system whereby the primary laser would emit at 365 and 488 nm, and excite CB, FITC, PE and PE-TR tandem, while the secondary laser is emitting at 633 nm exciting APC and the APC-Cy7 tandem.
REFERENCES
Horan PK, Muirhead KA, Slezak SE. Standards and controls in flow cytometry. In: Melamed MR, Lindmo T, Mendelsohn ML, editors. Flow cytometry and sorting. 2nd ed. New York: Wiley-Liss 1990.
Roederer M, Kantor AB, Parks DR, Herzenberg LA. Cy7PE and Cy7APC: bright new probes for immunofluorescence. Cytometry 1996;24:191-197.
Shapiro HM. Practical flow cytometry. 3rd ed. New York: Wiley 1994.
Stewart CC. Multiparameter analysis of leukocytes by flow cytometry. In: Darzynkiewicz Z, Crissman HA, editors. Flow Cytometry. San Diego: Academic Press, 1990:427-450.
Waggoner AS. Fluorescent probes for cytometry. In: Melamed
MR, Lindmo T, Mendelsohn ML, editors. Flow cytometry and sorting. 2nd ed.
New York: Wiley-Liss 1990.
