

Energy released during oxidation reactions in the mitochondrial respiratory chain is stored as an electrochemical gradient consisting of a transmembrane electrical potential (D y ), negative inside of about 180-200 mV, and a proton gradient of about 1 unit; this energy is then able to drive the synthesis of ATP to fuel the cell's energy dependent processes. Membrane permeable lipophilic cations, accumulated by living cells, organelles and liposomes exhibiting a negative interior membrane potential, have been used to decipher mechanisms regulating energy transduction and its control. Such probes include those which exhibit optical and fluorescence activity after accumulation into energized systems [i.e., 3,3'-diehexiloxadicarbocyanine iodide, nonylacridine orange (NAO), safranine O, rhodamine-123 (Rh123) etc.], radiolabelled probes, (i.e., [3H]methyltriphenyl-phosphonium, etc.) and unlabelled probes used with specific electrodes [i.e., tetraphenyl-phosphonium ion (TPP+) etc.].
These systems have several possible disadvantages, including the: a) time required to achieve equilibrium distribution of a mitochondrial membrane probe; b) degree of passive (unspecific) binding of probes to a membrane component, such as in the case of NAO, which detects mitochondrial mass as it binds to cardiolipin [1], or Rh123, which has several energy-independent binding sites [2]; c) toxic effects of probes on mitochondrial functional integrity; d) sampling procedures; e) interference from light scattering changes and from absorption changes of mitochondrial components; f) requirement of large amounts of biological materials. TPP electrode affords an easy and precise tool to measure D y due to the: i) low interference between bound TPP+ and the membrane; and ii) lack of responses of the electrode to species different from TPP+. However, this method requires discrete amounts of biological samples and, in contrast to isolated mitochondria, uptake of this lipophilic cation by several intact mammalian cells is a slow process.
Recently, we have developed a new cytofluorimetric (FCM) technique by using the lipophilic cation 5,5',6,6'tetrachloro-1,1',3,3'-tetraethylbenzimidazol-carbocyanine iodide (or, simply, JC-1) to detect variations in D y at the single cell level [3]. The method has been further validated at the single mitochondrion level [4]. JC-1 is more advantageous over rhodamines and other carbocyanines, capable of entering selectively into mitochondria, since it changes reversibly its color from green to orange as membrane potentials increase (over values of about 80-100 mV). This property is due to the reversible formation of JC-1 aggregates upon membrane polarization that causes shifts in emitted light from 530 nm (i.e., emission of JC-1 monomeric form) to 590 nm (i.e., emission of J-aggregate) when excited at 490 nm [5,6]. Thus, the color of the dye changes reversibly from green to greenish orange as the mitochondrial membrane becomes more polarized [7]. Both colors can be detected using the filters commonly mounted in all flow cytometers, so that green emission can be analyzed in fluorescence channel 1 (FL1) and greenish orange emission in channel 2 (FL2). The main advantage of the use of JC-1 is that it can be both qualitative, considering the shift from green to orange fluorescence emission, and quantitative, considering the pure fluorescence intensity, which can be detected in both FL1 and FL2 channels. For example, if you stain freshly collected peripheral blood mononuclear cells, you can easily distinguish the fluorescence signal coming from lymphocytes from that of monocytes, as monocytes give you a much higher signal, either in FL-1 or FL-2 (Figure 1). Clearly, this is because they have more organelles.
First Tip: never say that monocytes - or their
mitochondria - are "hyperpolarized", because mitochondria cannot go over
their maximal D y
! This word has already provoked tremendous and unexpected reactions from
the experts in bioenergetics in several meetings......
Probably all the readers have done more complicated things. The basic assumption is that if a cell has mitochondria, the technique should work - according to Murphy’s laws, until you try to do it.
In any case, if you are dealing with lymphocytes, treat a culture as you like (e.g. induce apoptosis, damage cells with drugs, H2O2, etc.), then take a cell suspension, adjust it to a density of 0.5-1x106 cells/mL and incubate in complete medium (RPMI 1640 with 10% FCS is OK, but I have tried many other culture media, that work as well) for at least 10-15 min. at room temperature in the dark with 10 µg/mL JC-1. Incubation can also be done in a 37°C incubator, for 10 min. The temperature and length of incubation depend on the cell type, and you have to set up the right conditions. A little bit of serum during the staining is recommended - cells feel much better.
Note that JC-1 has to be dissolved and stored according to the manufacturer's instruction, i.e. in DMSO or DMF. Be careful: vortex well during the addition of JC-1 or immediately after, as the solubility of the probe is very low in water. This is a fundamental trick, and following this suggestion you will not see horrible little coloured things, which are aggregates of the probe. At the end of the incubation period cells have to be washed twice in PBS (or what you like), resuspended in a total volume of 400 µL PBS and analyzed.
It is nice to prepare always what I call a functional, negative control, to be sure that the probe is working well. If you treat cells with drugs able to collapse DY, such as the K+ ionophor valinomycin (100 nM or more) or the proton translocator carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP, 250 nM), you should see a dramatic change of the fluorescence distribution. A typical example is shown in Figure 2. U937 human monoblastic leukemic cells were first treated with 100 nM valinomycin (10 min. at 37°C) and then stained with JC-1. Note the relevant shift to the lower right part of the quadrants. It is important to remind that each cell culture can have a small amount of dead or suffering cells. You can easily recognize these cells in the right lower part of the left panel. In this case they were less than 4%.
Figure 3 represents the intracellular events that occur when you stain cells with JC-1, and when a fall in D y is provoked by the incubation with valinomycin.
Another good control is that of mitochondrial mass, that
can be done with nonyl acridine orange (NAO), a probe capable
to bind mitochondria independently of their energization state [1], whose
fluorescence is detectable in FL1. Typically, cells are incubated at the
concentration of 0.5-1x106 cells/mL with 10 µM NAO for
10 min. in the dark at room temperature, washed twice in cold PBS and immediately
analyzed. The result you obtain gives you an idea of the mass of mitochondria
present within a cell, and allows you to be sure that the changes you see
with a potential-sensitive probe are not dependent upon the simple loss
of organelles. Referees like it.
Actually, I am using a FACScan (Becton Dickinson, San Josè, USA) equipped with a single 488 nm argon laser. The filter in front of the FL1 photomultiplier (PMT) transmits at 530 nm and has a bandwidth of 30 nm, the filter used in FL2 channel transmits at 585 and has a bandwidth of 42 nm. For the analysis of cells stained with JC-1, the PMT value of the detector in FL1 is set at about 360 V, FL2 PMT at 310 V; FL1-FL2 compensation about 4.0%, FL2-FL1 compensation 12.0%. I always take a minimum of 10-15,000 cells per sample, acquired in list mode with Lysys II software (Becton Dickinson). For the analysis of the files, I use Lysys II or a splendid software that works under Windows 3.1 or Windows 95, i.e. WinMDI (written by Dr. Joe Trotter, Scripps Research Institute, La Jolla, CA, USA). You can download it (for free!) through the Internet site <http://www.bio.umass.edu/mcbfacs/flowcat.html>.
Second Tip: the fluorescence signal of JC-1 aggregates goes also in FL-3, but is quite low. Thus, it is possible to use saturating concentrations of a mAb conjugated with a bright fluorochrome that emits in the deep red to recognize, for example, different lymphocyte subpopulations. The trick is to keep very low PMT values either in FL2 or FL3, and, obviously, to compensate well until you see a narrow, distinct peak in FL3 (this is the mAb). Unfortunately, it is not possible to use propidium iodide for simultaneous measurements of cell viability.
Third Tip (an expensive one): buy a FACS with another laser that can excite fluorochromes like Hoechst. By the way, I have used a triple laser Elite, from Coulter (in Paris, France). It had a 488 nm and an UV laser, with which it was possible to detect D y and glutathione content (with monobromobimane) in the same cell. Other experiments were done with JC-1 and allophycocyanin-conjugated mAbs, excited with a laser working at 633 nm.
Concerning instruments, I have used also an Excel, from
Coulter (in Bergen, Norway), a FACSTAR (here at home) and a FACSCalibur,
from Becton Dickinson (in Krakow, Poland), a Biorad Brite and a Partec
(in Krakow too), and they work perfectly as well. Obviously, compensations
have to be set according to your feelings.
Clearly, D y has been previously studied by FCM techniques, mostly by evaluating the changes in fluorescence of different dyes. Researchers used first Rh123 [8,9], then other molecules such as 3,3’-dihexiloxocarbocyanine iodide [DiOC6(3)] [10]. Typically, the signal coming from cells whose mitochondria had a low potential was much lower than that of control samples, and in a classical histogram depolarized populations go to the left. As after the shift to the left the peaks (i.e. that of controls and treated cells) are not always perfectly separate, the operator has to decide where the population of cells with depolarized mitochondria begins. These two fluorescent probes have this and other problems, and we have spent some time in analyzing them, and in understanding which is their correct use.
Rh123 binding to mitochondria is difficult to calculate when the cell presents a certain mitochondrial heterogeneity due, for example, to a high number of mature or immature organelles, as occurs in a continuously growing cell line. Moreover, it has been reported that in rat liver mitochondria different binding sites for Rh123 exist, i.e. sites which are freely accessible whatever the energy status of the mitochondria and sites which are hidden in the energized state and freely accessible in the deenergized form of the organelle [2]. This has been attributed to different maturative states of the organelles. Thus, in a single cell, organelles can have different Rh123 binding sites with consequent different fluorescence emissions. As a result, it is very difficult to ascertain whether or not mitochondria bind Rh123 in an energy-dependent or energy-independent manner. However, the probe is perfect when used in association with propidium iodide, as this combination allows a clear and elegant distinction between dead and living cells.
DiOC6(3) is more reliable for analysis of plasmamembrane potential rather than for studies on DY. Indeed, the first application of this probe in FCM was for the analysis of plasmamembrane potential [11]. After this, DiOC6(3) was used in isolated mitochondria to detect D y changes [10]. Obviously, it worked: any cationic molecule goes to negative sites, and is released when the charge changes. If that molecule is fluorescent, the signal decreases when the membrane potential of the organelle is lost. The problems start when intact cells are used. In our hands, DiOC6(3) reacted properly when U937 cells were treated with FCCP, but such behaviour was not observed in cells treated with valinomycin. Moreover, when cells were kept in the presence of plasmamembrane depolarizing agents such as ouabain or high doses of extracellular K+, a consistent decrease in DiOC6(3) fluorescence was noted, indicating a consistent sensitivity of the probe for plasmamembrane (Figures 4 and 5).
This behaviour was not totally unexpected, as it is known that this probe can bind several membranes other than mitochondria [12], as also reported in the Handbook of Fluorescent Probes and Research Chemicals (edited by the Company that produces and sells this reagent, i.e. Molecular Probes, Eugene, OR, USA). Thus, using this probe, it is very difficult, if not impossible, to distinguish between depolarization of plasmamembrane or changes in DY in several physiological or pathological conditions, when both events can take place.
Figure 6 shows that DiOC6(3) can go into mitochondria, but after the fall in D y a peculiar intracellular distribution can take place. Indeed, the probe can redistribute to other membranes such as those of the endoplasmic reticulum (ER), that have a negative charge. The result is that the cell is still fluorescent, and the fluorescence signal can maintain the same intensity. Similar problems are avoided by the use of a probe like JC-1, that changes color after the de-aggregation provoked by the fall in D y .
Surprisingly enough, DiOC6(3) has been widely
used to detect D y
changes during apoptosis [13-18], and a theory has been put forward stating
that loss of D y
is an early event - if not the cause - of programmed cell death [19]. The
methodological analysis of the fluorescence distribution of DiOC6(3),
together with several reports from our and other groups [20-26], cast some
doubts on those studies which, using a PMP-sensitive probe such as DiOC6(3)
for analyzing DY, generalized that mitochondria
depolarization is one of the first events occurring during apoptosis.
The technique is routinely used by us and by many groups whose members had the possibility to visit our laboratory. Clearly, all scientists who are interested in these studies and in JC-1 technique are invited to contact us, and are very welcome. In the last years, the use of JC-1 allowed us to demonstrate:
ii) that N-acetyl-cysteine protects human cells from TNF-induced apoptosis through an action that also involves mitochondria [22];
iii) the role of mitochondria in the cytotoxic damage induced by L-histidine, whose action is enhanced by inorganic or organic hydroperoxide [28];
iv) the different composition in mitochondria mass and different sensitivity to depolarizing agents of small and large coelomocytes from the earthworm Eisenia foetida [21];
v) the toxicity at the mitochondrial level of the doses of chromium which are regularly used by most researchers in studies on NK cell functions [29];
vi) that mitochondria are a target for the protective effects of heat shock proteins after an oxidative stress [30];
vii) that mitochondrial membrane potential can be modified by the action of cytokines such as TNF-a [31];
viii) that mitochondrial functional alterations and a dramatic tendency to undergo apoptosis are characteristics of lymphocytes obtained from patients experiencing and acute, primary HIV syndrome [24], where a variety of immunological alterations take place [32,33];
ix) that mitochondria functionality is well preserved in healthy centenarians [34], i.e. individuals able to reach the extreme limit of human life with an intact immune system [35-39].
I am grateful to all the scientists with whom I had the
pleasure to collaborate in these years, and especially to those more involved
in the ongoing mitochondrial adventure. In particular, I express gratitude
and appreciation to the help and patience of Professors Claudio Franceschi,
Edwin L. Cooper, Walter Malorni, Alberto Masini, Valentina Bobyleva, Barbara
S. Polla, Christoph Richter and Umberto Muscatello, and of Drs. Stefano
Salvioli, Daniela Monti, Miriam Capri, Daniela Barbieri, Sabrina Macchioni,
Andrea Ardizzoni, Bård Røsok.
2. Lopez-Mediavilla C., Orfao A., Gonzales M., Medina J.M. Identification by flow cytometry of two distinct rhodamine-123-stained mitochondrial populations in rat liver. FEBS Lett., 254: 115-120, 1989.
3. Cossarizza A., Baccarani Contri M., Kalashnikova G., Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem. Biophys. Res. Commun., 197: 40-45, 1993.
4. Cossarizza A., Ceccarelli D., Masini A. Functional heterogeneity of isolated mitochondrial population revealed by cytofluorimetric analysis at the single organelle level. Exp. Cell Res., 222: 84-94, 1996.
5. Hada H., Honda C., Tanemura H. Spectroscopic study on the J-aggregate of cyanine dyes. I. Spectral changes of UV bands concerned with J-aggregate formation. Photogr. Sci. Eng., 21: 83-91, 1977.
6. Reers M., Smith T.W., Chen L.B. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry, 30: 4480-4486, 1991.
7. Smiley S.T., Reers M., Mottola-Hartshorn C., Lin M., Chen A., Smith T.W., Steele G.D., Chen L.B. Intracellular heterogeneity in mitochondrial membrane potential revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. USA, 88: 3671-3675, 1991.
8. Johnson L.V., Walsh M.L., Chen L.B. Localization of mitochondria in living cells with rhodamine 123. Proc. Natl. Acad. Sci. USA, 77: 990-994, 1980.
9. Darzynkiewicz Z., Staiano-Coico L., Melamed M.R. Increased mitochondrial uptake of rhodamine 123 during lymphocyte stimulation. Proc. Natl. Acad. Sci. USA, 78: 2383-2387, 1981.
10. Petit P.X., O'Connor D., Grunwald D., Brown S.C. Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications. Eur. J. Biochem., 194: 389-397, 1990.
11. Jenssen H.-L., Redmann K., Mix E. Flow cytometric estimation of transmembrane potential of macrophages - A comparison with microelectrode measurements. Cytometry, 7: 339-346, 1986.
12. Terasaki M., Song J., Wong J.R., Weiss M.J., Chen B.L. Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell, 38: 101-108, 1984.
13. Vayssière J.L., Petit P.X., Risler Y., Mignotte B. Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40. Proc. Natl. Acad. Sci. USA, 91: 11752-11756, 1994.
14. Zamzami N., Marchetti P., Castedo M., Zanin C., Vayssiere J.-L., Petit P.X., Kroemer G. Reduction in mitochondrial potential constitutes and early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med., 181: 1661-1672, 1995.
15. Zamzami N., Marchetti P., Castedo M., Decaudin D., Macho A., Hirsch T., Susin S.A., Petit P.X., Mignotte B., Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med., 182: 367-377, 1995.
16. Macho A., Castedo M., Marchetti P., Aguilar D., Zamzami N., Girard P.M., Uriel J., Kroemer G. Mitochondrial dysfunctions in circulating T lymphocytes from human immunodeficiency virus-1 carriers. Blood, 86: 2481-2487, 1995.
17. Petit P.X., Lecoeur H., Zorn E., Dauguet C., Mignotte B., Gougeon M.-L. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J. Cell Biol., 130: 157-167, 1995.
18. Kroemer G., Petit P., Zamzami N., Vayssiere J.L., Mignotte B. The biochemistry of programmed cell death. FASEB J., 9: 1277-1287, 1995.
19. Kroemer G., Zamzani N., Susin S.A. Mitochondrial control of apoptosis. Immunol. Today, 18: 44-51, 1997.
20. Cossarizza A., Kalashnikova G., Grassilli E., Chiappelli F., Salvioli S., Capri M., Barbieri D., Troiano L., Monti D., Franceschi C. Mitochondrial modifications during rat thymocyte apoptosis: a study at the single cell level. Exp. Cell Res., 214: 323-330, 1994.
21. Cossarizza A., Cooper E.L., Quaglino D., Salvioli S., Kalachnikova G., Franceschi C. Mitochondrial mass and membrane potential in coelomocytes from the earthworm Eisenia foetida: studies with fluorescent probes in single intact cells. Biochem. Biophys. Res. Commun., 214: 503-510, 1995.
22. Cossarizza A., Franceschi C., Monti D., Salvioli S., Bellesia E., Rivabene R., Biondo L., G. R., Tinari A., Malorni W. Protective effect of N-acetylcysteine in Tumor Necrosis Factor a-induced apoptosis in U937 cells: the role of mitochondria. Exp. Cell Res., 220: 232-240, 1995.
23. Cossarizza A., Salvioli S., Franceschini M.G., Kalashnikova G., Barbieri D., Monti D., Grassilli E., Tropea F., Troiano L., Franceschi C. Mitochondria and apoptosis: a cytofluorimetric approach. Fund. Clin. Immunol., 3: 67-68, 1995.
24. Cossarizza A., Mussini C., Mongiardo N., Borghi V., Sabbatini A., De Rienzo B., Franceschi C. Mitochondria alterations and dramatic tendency to apoptosis in peripheral blood lymphocytes during acute HIV syndrome. AIDS, 11: 19-26, 1997.
25. Yang J., Liu X., Bhalla K., Kim C.N., Ibrado A.M., Cai J., Peng T.I., Jones D.P., Wang X. Prevention of apoptosis by bcl-2: release of cytochrome c from mitochondria blocked. Science, 275: 1129-1132, 1997.
26. Kluck R.M., Bossy-Wetzel E., Green D.R., Newmeyer D.D. The release of cytochrome c from mitochondria: a primary site for bcl-2 regulation of apoptosis. Science, 275: 1132-1136, 1997.
27. Richter C., Schweizer M., Cossarizza A., Franceschi C. Control of apoptosis by the cellular ATP level. FEBS Lett., 378: 107-110, 1996.
28. Guidarelli A., Sestili P., Cossarizza A., Franceschi C., Cattabeni F., Cantoni O. Evidence for dissimilar mechanisms of enhancement of inorganic and organic hydroperoxide cytotoxicity by L-histidine. J. Pharmacol. Exp. Therap., 275: 1575-1582, 1995.
29. Borella P., Bargellini A., Salvioli S., Incerti Medici C., Cossarizza A. The use of non-radioactive chromium as an alternative to 51Cr in NK assays. J. Immunol. Methods, 186: 101-110, 1995.
30. Polla B.S., Kantengwa S., François D., Salvioli S., Franceschi C., Marsac C., Cossarizza A. Mitochondria as targets for the protective effects of heat shock against oxidative injury. Proc. Natl. Acad. Sci. USA, 93: 6458-6463, 1996.
31. Polla B.S., Kantengwa S., Marièthoz E., Jacquier-Sarlin M., Hennet T., Russo-Marie F., Cossarizza A. TNF-a alters mitochondrial membrane potential in L929 but not in TNF-a-resistant L929.12 cells: relationship with the synthesis of heat shock proteins and superoxide dismutase activity. Free Rad. Res., 25: 125-131, 1996.
32. Cossarizza A., Ortolani C., Mussini C., Borghi V., Guaraldi G., Mongiardo N., Bellesia E., Franceschini M.G., De Rienzo B., Franceschi C. Massive activation of immune cells with an intact T cell repertoire in acute HIV syndrome. J. Infect. Dis., 172: 105-112, 1995.
33. Cossarizza A., Ortolani C., Mussini C., Guaraldi G., Mongiardo N., Borghi V., Barbieri D., Bellesia E., Franceschini M.G., De Rienzo B., Franceschi C. Lack of selective Vb deletion in CD4+ or CD8+ T lymphocytes and functional integrity of T cell repertoire during acute HIV syndrome. AIDS, 9: 547-554, 1995.
34. Cossarizza A., Ortolani C., Monti D., Franceschi C. Cytometric analysis of immunosenescence. Cytometry, 27: 297-313, 1997.
35. Sansoni P., Cossarizza A., Brianti V., Fagnoni F., Snelli G., Monti D., Marcato A., Passeri G., Ortolani C., Forti E., Fagiolo U., Passeri M., Franceschi C. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood, 80: 2767-2773, 1993.
36. Franceschi C., Cossarizza A. Introduction: the reshaping of immune system with age. Int. Rev. Immunol., 12: 1-3, 1995.
37. Franceschi C., Monti D., Barbieri D., S. S., Negro P., Capri M., Guido M., Azzi R., P. S., Paganelli R., Fagiolo U., Baggio G., Donazzan S., Mariotti S., D'Addato S., Gaddi A., Ortolani C., Cossarizza A. Immunosenescence in humans: deterioration or remodelling? Int. Rev. Immunol., 12: 57-74, 1995.
38. Cossarizza A., Barbieri D., Londei M. T cell repertoire usage in humans, from newborns to centenarians. Int. Rev. Immunol., 12: 41-55, 1995.
39. Franceschi C., Monti D., Sansoni P., Cossarizza A.
The immunology of exceptional individuals: the lesson of centenarians.
Immunol. Today, 16: 12-16, 1995.
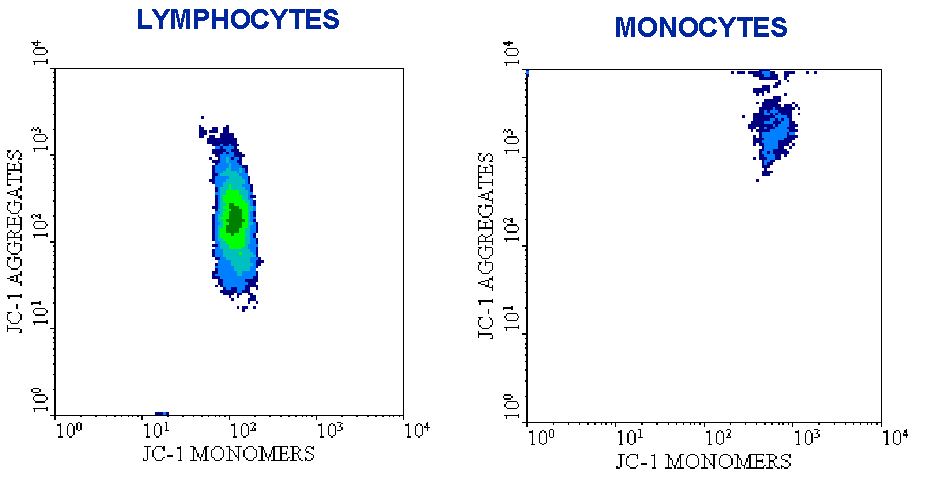
Figure 1. JC-1 staining of peripheral blood
lymphocytes and monocytes. Note the different fluorescence intensity of
the two cell types, due to the presence of a higher number of mitochondria
in monocytes.
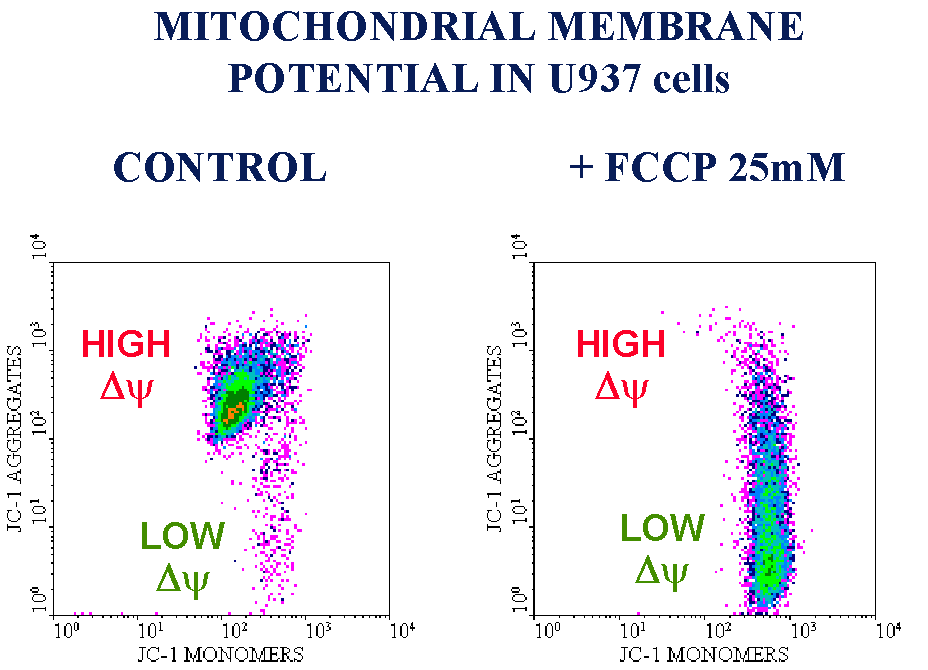
Figure 2. Effects of the incubation with
the depolarizing agent valinomycin on D y
. Note the relevant shift towards the lower right part of the panel.
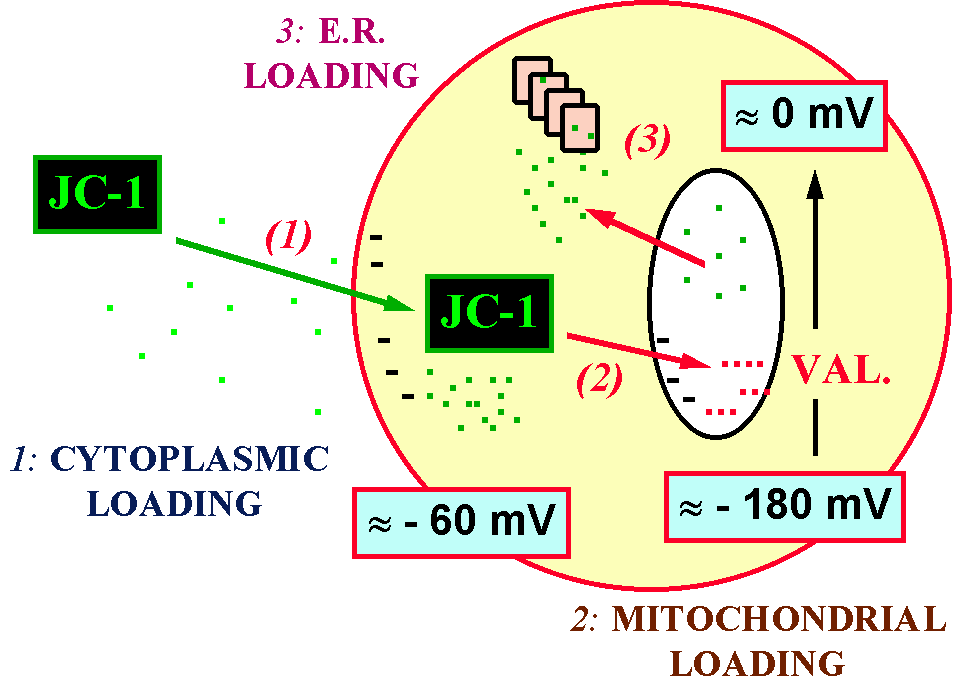
Figure 3. Intracellular distribution of JC-1
before and after the depolarization of mitochondria with valinomycin (VAL.).
Note that 3 events occur: 1) cytoplasmic loading, which is dependent upon
plasmamembrane potential; 2) mitochondrial loading, dependent upon D
y ; 3) endoplasmic reticulum (E.R.) loading,
that theoretically can take place after the fall in D
y . In the case of JC-1, aggregates are no more
present when the dye is released from mitochondria.
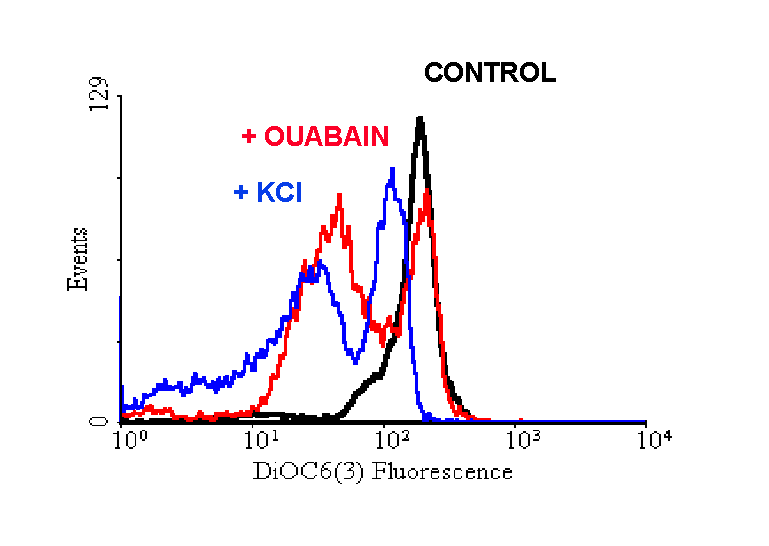
Figure 4. Effects of ouabain and of the
incubation in a K+-rich medium on the fluorescence intensity
of U937 cells stained with DiOC6(3). Note the extreme sensitivity
of the probe to agents that depolarize plasmamembrane but do not affect
D y .
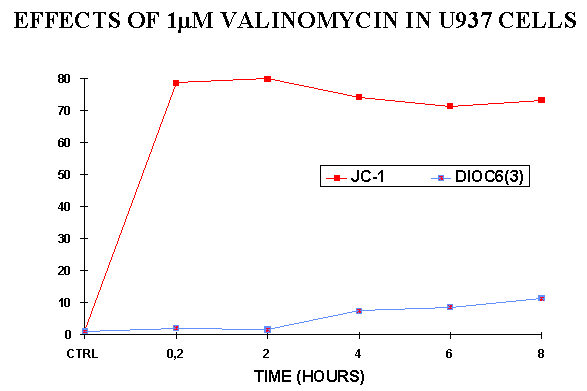
Figure 5. Comparison between the fluorescence
of JC-1 and DiOC6(3) after the treatment of U937 cells with
valinomycin, that collapses D y
. Note that in this case DiOC6(3) is not sensitive to the loss
of mitochondrial membrane potential. In ordinate: % of cells whose fluorescence
indicates the depolarization of mitochondria.
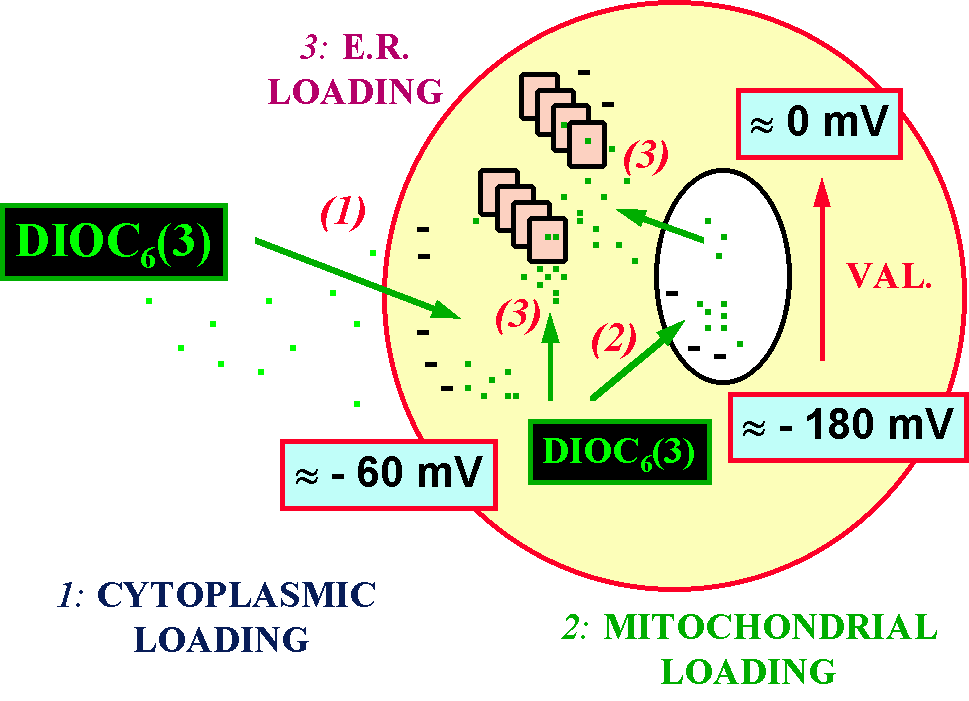
Figure 6. Intracellular distribution of
DiOC6(3) before and after the depolarization of mitochondria
with valinomycin (VAL.). Note that also in this case 3 events can occur:
1) cytoplasmic loading, which is dependent upon plasmamembrane potential;
2) mitochondrial loading, dependent upon D y
; 3) endoplasmic reticulum (E.R.) loading, that theoretically can take
place either after the fall in D y
or after the cytoplasmic loading. This model can explain the apparent lack
of effects of valinomycin on U937 cells stained with this probe, as the
fluorescence signal that is produced within the cell is poorly related
to Dy values.
